FDA Approves Double-Chin Eliminator Injection, Kybella
Here's what to know the drug that claims to get rid of your double chin.
— -- The Food and Drug Administration today approved a new drug that promises to get rid of double chins without surgery.
The drug is called Kybella, and it is an injectable substance that dissolves fat under the chin. It is expected to be commercially available in June, according to the company.
"Options at the moment for submental fat [double chins] are [to] cut it out or suck it out," Dr. Susan Weinkle, a dermatologist in Florida who has been working with the drug in trials since 2007, told ABC News in March when the drug was poised for approval. "However, this is going to be a noninvasive in-the-office procedure that can be performed by your dermatologist and [gets] excellent results."
Here's what you need to know:
What is it?
Kybella is a version of deoxycholic acid, "a naturally occurring molecule in the body that aids in the breakdown of dietary fat," according to its manufacturer, Kythera Biopharmaceuticals, based in Westlake Village, California. The company had been calling it ATX-101 before deciding to name it Kybella.
How does it work?
"It disrupts the fat cell," said Dr. Derek Jones, who gave Kythera's presentation to the FDA in March. "When it disappears, it disappears permanently."
Jones said that the drug destroys the fat cell's membrane, causing it to burst. What remains of the fat cell is absorbed back into the body "via normal metabolic pathways," he said.
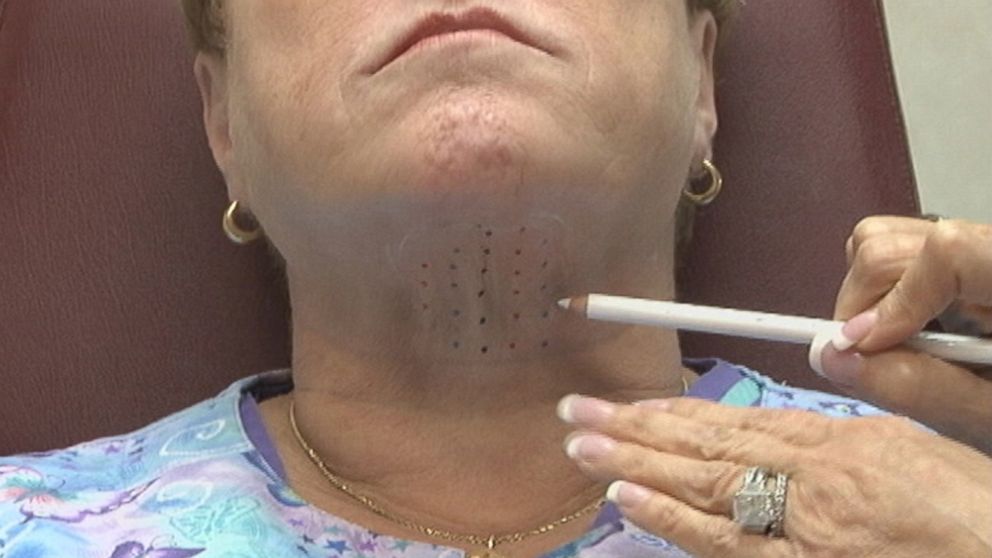
How much is needed?
Weinkle draws a grid of tiny dots beneath her patient's chin, she said, and injects 0.2 cubic centimeters into each dot.
"I actually mark the area that I see the max amount of fat," she said.
How long does it take?
It takes her five minutes to do the injections, she said, adding that she applies a little ice beforehand.
What's recovery like?
It takes two or three days to heal, Weinkle said. And no bandages are required.
How thoroughly has it been studied?
According to Kythera, it's been the subject of 19 clinical studies involving 2,600 patients.
Dr. William Stebbins, a dermatologist at Vanderbilt University Medical Center who was not involved in the studies, said he has seen Kythera's presentations at various dermatological meetings.
"I've been hearing about this for years, and I'm actually really glad it's coming out," he said.
Who isn't a good candidate for it?
"This is not necessarily a silver bullet that will solve the problem for every patient, but it certainly is a good option for appropriate candidates," said Dr. Joshua Zeichner, a dermatology professor at the Mount Sinai Hospital in Manhattan. He has not been involved in the drug's development.
People with a lot of excess skin under their chin and neck aren't good candidates for this drug, he said.
"That's not a fat problem," he said. "It’s a skin problem."
Are there side effects?
Yes, side effects were bruising, swelling and temporary numbness, Stebbins said.
Will insurance cover it?
Insurance will not cover this procedure, and it's too soon to say how much it will cost, Zeichner said. In trials, patients received the injections once a month for up to six months before reaching the desired effect.
Why can't we use this in other areas of the body?
Jones said it would take significantly more product to remove fat from other areas of the body, and liposuction and other options would be more efficient.




