Controversial coronavirus drug hydroxychloroquine administered at 2 nursing homes, senators say
Democratic senators want to know what's been done to stop unproven treatment.
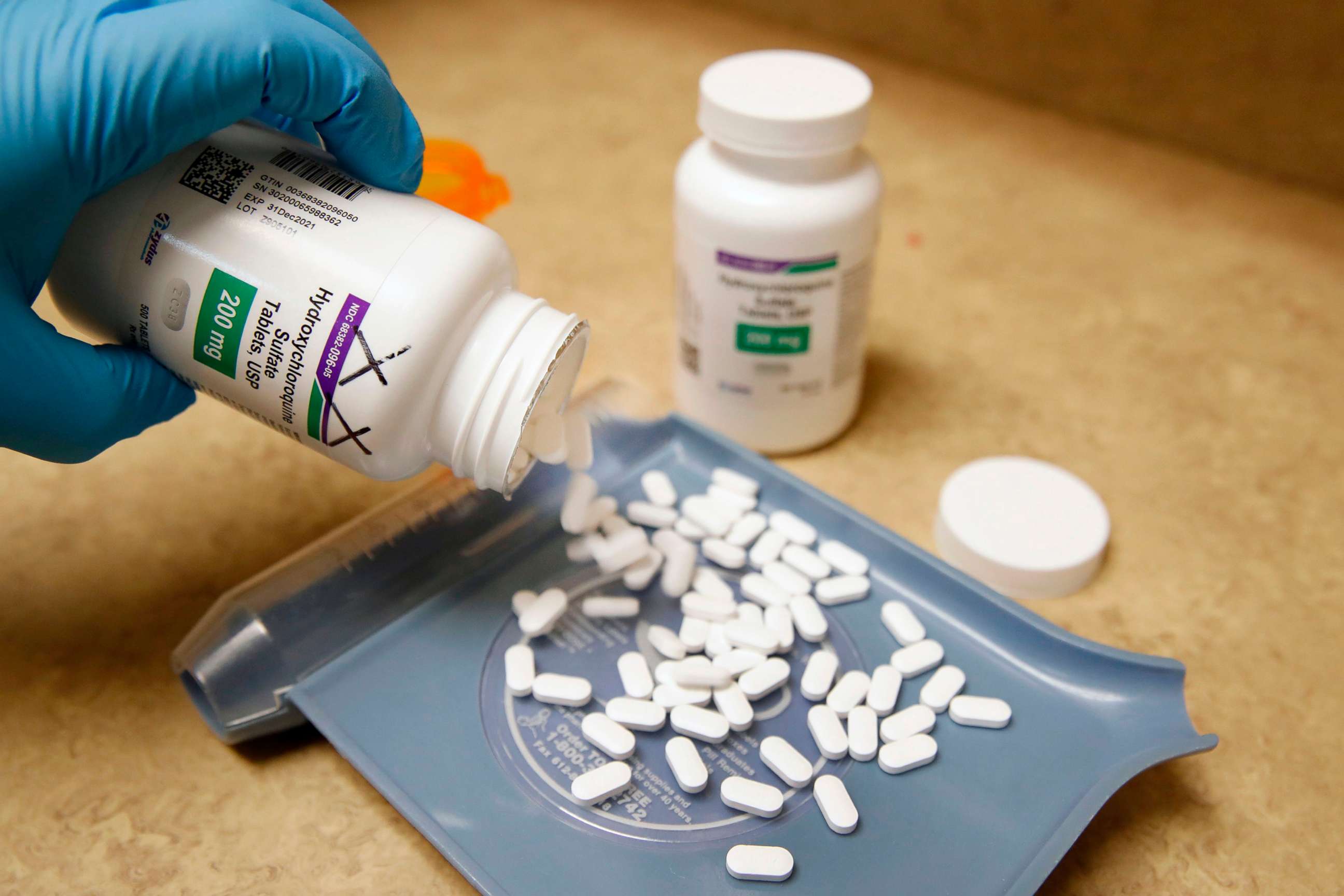
Nursing homes in Texas and Pennsylvania administered hydroxychloroquine to residents battling novel coronavirus without first gaining needed approvals, according to state inspector reports highlighted by a group of Democratic senators Thursday.
The senators said the drug, which has been aggressively touted by President Donald Trump as a promising treatment for COVID-19 despite sparse evidence, was used in one nursing home after the Food and Drug Administration specifically warned against its use in non-hospital settings.
Sens. Elizabeth Warren, D-Mass., Bob Casey, D-Pa., and Ron Wyden, D-Ore., said they found the reports citing two separate incidents, which impacted over 200 patients, "concerning" and sent letters Thursday to federal agencies that regulate nursing homes across the U.S. They requested details on what efforts have been made to insure nursing home residents are not being subjected to unproven or unsafe treatments for the virus.
"The use of hydroxychloroquine is all the more concerning due to warnings from medical experts about the increased risks seniors face from the drug," the senators wrote.
The Centers for Medicare and Medicaid, the federal agency tasked with oversight of nursing homes, told ABC News they are carefully reviewing the letter and will respond directly to Warren, Casey and Wyden.
Hydroxychloroquine was touted by Trump during coronavirus briefings at the White House. The Food and Drug Administration initially gave emergency authorization for its use early in the pandemic. But mounting concerns about the drugs effectiveness in treating COVID-19 and risks to patients led to a reversal in April, when the FDA issued new warnings against prescribing hydroxychloroquine for use in non-hospital settings. In June, the FDA revoked emergency use authorization, citing evidence that the recommended dose is unlikely to be effective against the virus.
The Democratic senators raised concerns about incidents at facilities in Pennsylvania and Texas. At the Texas facility, inspectors found that treatment was administered without the informed consent of patients or their families. At the Pennsylvania facility, consent forms were signed but the state had not signed off.

"In a crisis, it's particularly concerning that a person wouldn't be given information about what drugs they're being given," said Patty Ducayet, the long-term care ombudsman for Texas Health and Human Services. "It almost feels like we collectively were in such a panic that rushing to treatments, it just becomes all the more important that people have good information before making a decision."
The three Democratic senators cited publicly available reports from the Pennsylvania Department of Health that found the Brighton Rehabilitation and Wellness Center in Beaver, Pennsylvania, had treated 205 of their 435 residents with hydroxychloroquine without approval from state officials.
"The facility failed to obtain the necessary approval from the Pennsylvania Department of Health prior to administering a medication that is not a generally accepted practice in the medical community," the inspector report founds.
These treatments continued even after the FDA issued its warning against use of the drug outside of a hospital, according to the report.
The senators also cited an incident at The Resort at Texas City indicating the nursing home had been treating cognitively impaired patients with "experimental medication" without the consent of family members.
While the state inspector report does not name hydroxychloroquine as the experimental medication, the lead physician at the same facility was featured in a Houston Chronicle report touting the use of hydroxychloroquine to treat an outbreak at The Resort.
ABC News did not get an immediate response to request for comment from either nursing facility.
Throughout the COVID-19 crisis, nursing homes have been a hotbed for the virus, with elderly and vulnerable patients succumbing to COVID at alarming rates. A recent survey of state-reported COVID-19 data found that at least 40% of all COVID-19 deaths in the United States have been nursing home residents.
Fatalities in these facilities remain high despite increased efforts from the Trump administration and individual facilities to deliver protective equipment and limit outside visitors that could spread the virus asymptomatically.
In their letters, the senators note that while they've documented two instances of patients being improperly treated with hydroxychloroquine, they fear that limited inspection and restricted visitation could mean there are even more cases that have gone unreported.
"While these were captured by the limited infection control and complaint inspections that have been taking place in nursing homes since the start of the pandemic, with regular inspections suspended and visitation by families and long-term ombudsman limited, it is possible that other instances like these have gone un-checked," the senators wrote.



