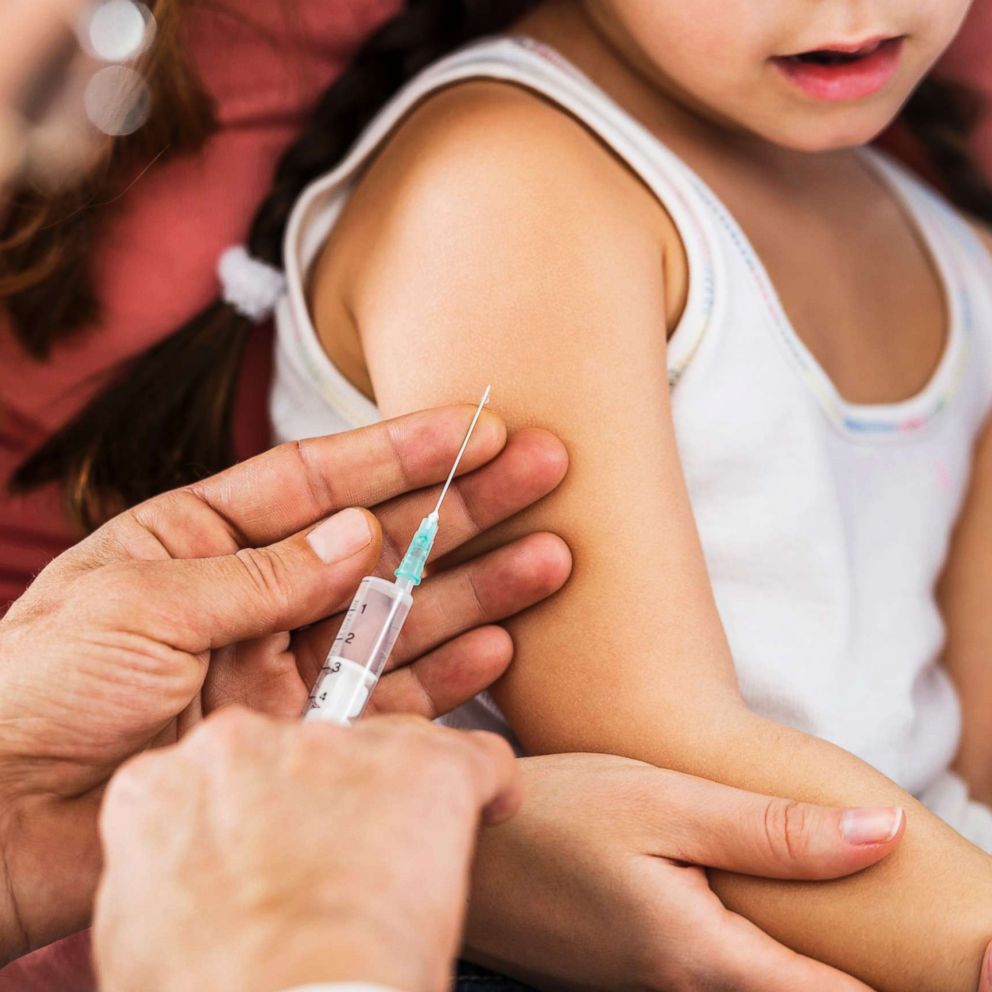
COVID-19 vaccines and kids: What parents should know
Kids as young as 6 months old are now eligible to get vaccinated against COVID.
Two years into the coronavirus pandemic, children as young as 6 months old are now eligible to get vaccinated against COVID-19.
The Centers for Disease Control and Prevention (CDC) on Saturday greenlit the Pfizer and Moderna COVID-19 vaccines for kids under 6, the last remaining age group to be eligible.
Here are nine questions answered about the COVID-19 vaccines and kids as families seek to make the best decisions.
1. What is the science behind the COVID-19 vaccine?
Both the Pfizer and Moderna vaccines use mRNA technology, which does not enter the nucleus of the cells and doesn't alter human DNA. Instead, it sends a genetic "instruction manual" that prompts cells to create proteins that look like the outside of the virus -- a way for the body to learn and develop defenses against future infection.
The Johnson & Johnson vaccine uses an inactivated adenovirus vector, Ad26, that cannot replicate. The Ad26 vector carries a piece of DNA with instructions to make the SARS-CoV-2 spike protein that triggers an immune response.
This same type of vaccine has been authorized for Ebola and has been studied extensively for other illnesses and for how it affects women who are pregnant or breastfeeding.
Neither of these vaccine platforms can cause COVID-19.
2. What is the status of vaccine eligibility for kids?
The Pfizer vaccine has already been available for those who are 5 and older and now is also available for kids ages 6 months through 4 years.
Moderna's vaccine has previously been available only for people ages 18 and older. The latest vaccine authorization is for kids ages 6 months to 5 years.
3. Why do kids need to be vaccinated against COVID-19?
While there have not been as many deaths from COVID-19 among children as adults, particularly adults in high-risk categories, kids can still get the virus and they can also transmit the virus to adults.
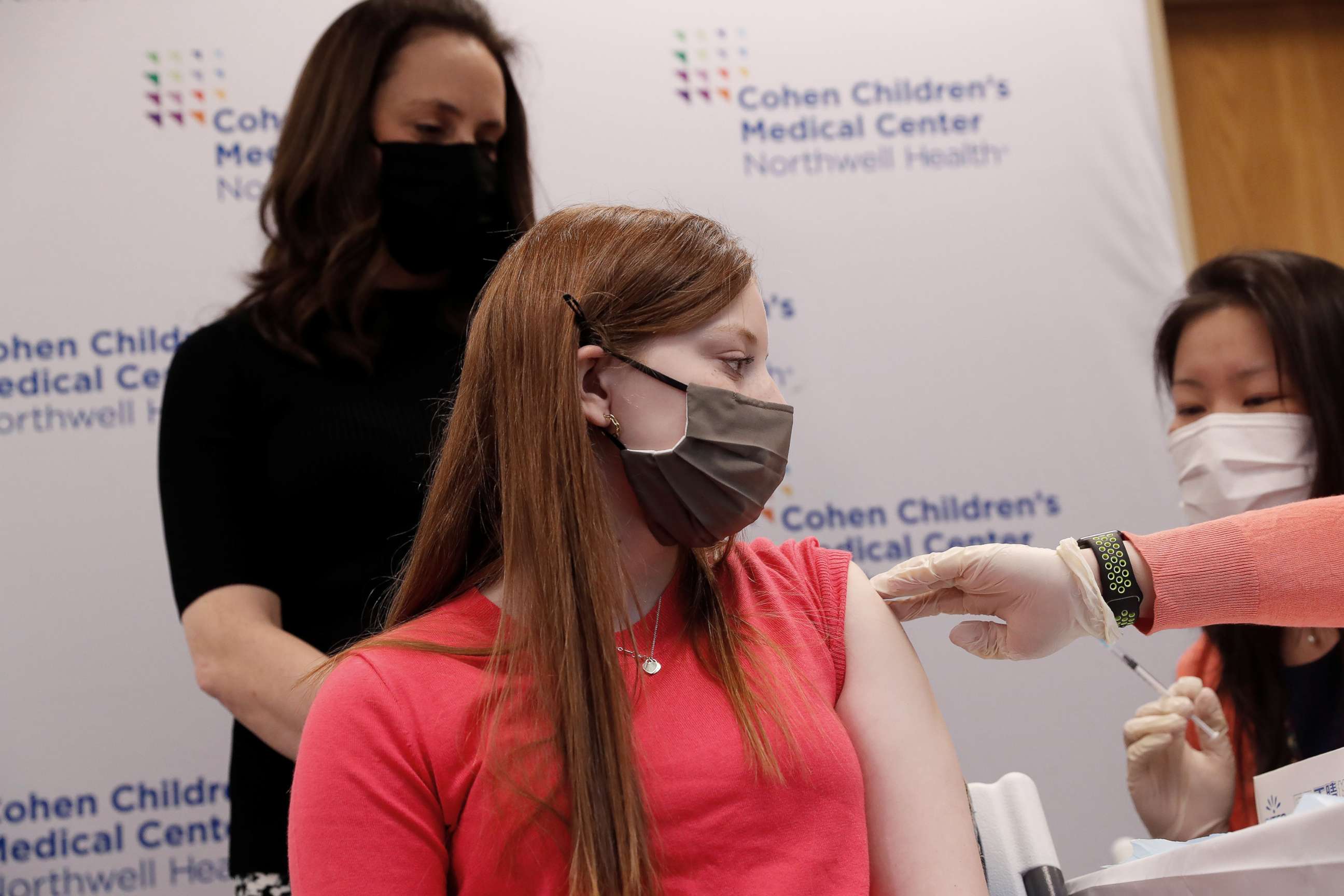
More than 13 million children have tested positive for the virus since the onset of the pandemic over two years ago, according to the American Academy of Pediatrics (AAP).
"One of the main issues is parents thinking that COVID-19 is a very mild disease, and the vaccines are very unsafe," Dr. Diego R. Hijano, pediatric infectious disease specialist at St. Jude Children's Research Hospital, told ABC News. "And we cannot emphasize that the opposite is true."
4. Do kids experience the same vaccine side effects as adults?
The side effects in children who get vaccinated against COVID-19 are typically mild, according to Dr. Vandana Madhavan, a pediatric infectious disease specialist at Mass General Hospital and professor at Harvard Medical School.
"The most common side effect from the COVID-19 vaccine in all age groups is a sore arm at the site of an injection," said Madhavan. "Children might get a low-grade fever, generalized fatigue and crankiness but these side effects are less common and self-resolved, going away in a couple of days."
5. Is there data showing COVID-19 vaccines are safe for kids?
The CDC released three studies in December showing COVID-19 vaccines are safe and effective for children.
One study, which evaluated the safety reports of more than 42,000 children ages 5 to 11 who received a Pfizer shot, found the side effects from the Pfizer vaccine were mostly mild and temporary. It also found that myocarditis, a heart inflammation side effect that has been associated with the mRNA vaccines in very rare cases, does not appear to be a risk.
A second study, which looked at data from 243 children ages 12 to 17 in Arizona, found the Pfizer vaccine was 92% effective at preventing infection. The study, conducted between July and December when delta was the dominant variant in the U.S., also found that adolescents who developed COVID-19 reported a lower percentage of time masked in school and time masked in the community.
The third study, also conducted when delta was dominant, found that among children ages 5 to 17 hospitalized due to COVID-19, less than 1% were fully vaccinated against the virus.
6. How effective are the vaccines in children?
Pfizer said its three-dose vaccine was 80% effective against symptomatic omicron COVID-19 infection among children 6 months to under 5 years old.
In March, the company said its clinical trials showed the vaccine was safe and 100% effective in children ages 12-15, similar to the 95% efficacy among adult clinical trial participants.
Moderna’s preliminary analysis found its two-dose pediatric vaccine was 51% effective against symptomatic COVID-19 among children 6 months to under 2 years old, and about 37% among children 2 to 5 years old -- roughly the same efficacy seen in adults during the omicron surge. Protection against serious disease and death was higher.
7. Do kids get the same dose of the vaccines as adults?
Pfizer's vaccine for kids under age 5 is three doses of 3 micrograms each. Each dose is one-tenth the adult dose.
Moderna's shot is two doses, with each dose a quarter of the adult dose. Moderna is also studying a third shot, or booster, for young children.
Kids ages 5 to 11 are given a 10-micrograms dose of the Pfizer vaccine, one-third of the adolescent and adult dose. Like with adults and adolescents, the pediatric vaccine is delivered in two doses, three weeks apart.
For 12-to-15-year-olds, the FDA has authorized the same dosing as adults with the Pfizer two-dose vaccine.
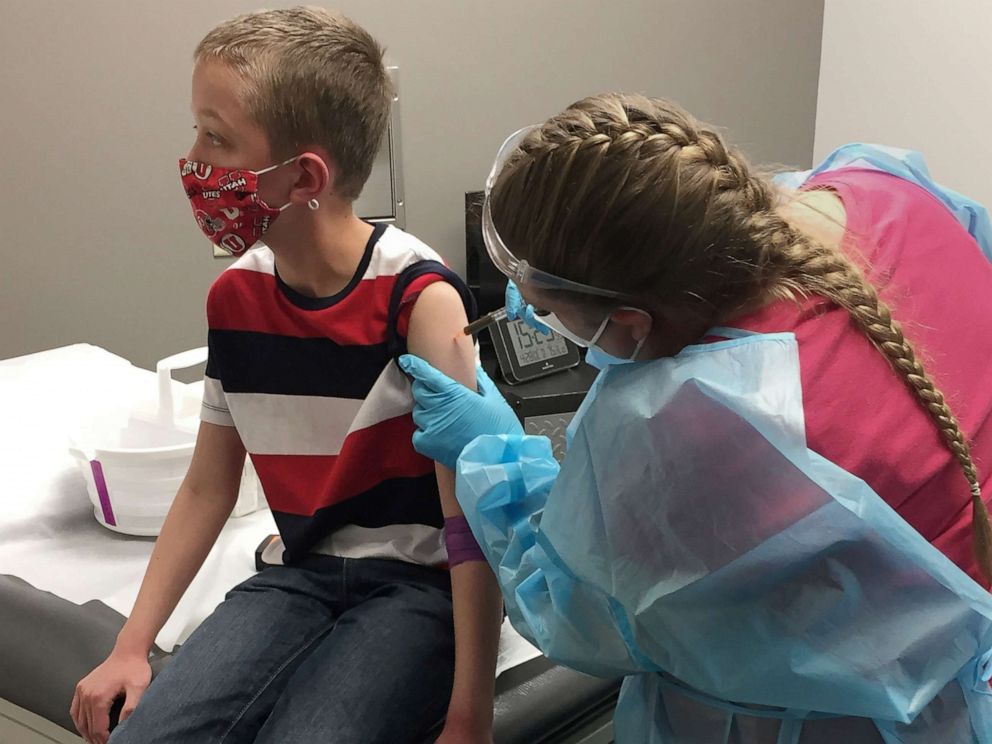
The FDA and CDC have recommended the Pfizer booster shots now available for kids ages 5 to 11 be administered five months after the primary vaccine series.
8. Could COVID-19 vaccines impact puberty and menstruation?
There is currently no clinical evidence to suggest any of the COVID-19 vaccines can have long-term effects on puberty or fertility.
9. Where can kids get vaccinated against COVID-19?
Vaccines are accessible at pediatricians' offices, children's hospitals, pharmacies like CVS, Walgreens and Rite-Aid and school and community-based clinics.
Parents can search for appointments at Vaccines.gov to find a local provider.
ABC News' Sasha Pezenik, Anne Flaherty, Eric Strauss, Cheyenne Haslett and Jade A. Cobern, MD, a member of the ABC News Medical Unit, contributed to this report.