Fall COVID-19 boosters could roll out soon pending green light from FDA, CDC
Experts are concerned about a potential viral resurgence this fall, winter.
With the end of summer nearing, and Labor Day around the corner, U.S. health officials are preparing to roll out millions of new COVID-19 boosters as health experts grow concerned over a potential viral resurgence in the fall and winter.
The Food and Drug Administration is expected, as soon as this week, to authorize Pfizer and Moderna's new bivalent booster shots, which target both the original Wuhan strain that emerged at the onset of the pandemic, as well as the omicron subvariants, BA.4 and BA.5, that are currently dominant globally.
Advisers to the Centers for Disease Control and Prevention (CDC) are set to meet on Thursday and Friday, and if both agencies greenlight the new shots, doses could be shipped out in the days to come and administered soon after Labor Day weekend.

Unlike the original vaccines and boosters, these new shots will not go through a lengthy clinical trial process, where thousands of Americans are dosed with the vaccines to test the safety and long-term effectiveness of the vaccines. However, federal health officials stress that these new shots will still be just as safe as the original vaccines because the underlying vaccine platform, mRNA, is the same.
"Real world evidence from the current mRNA COVID-19 vaccines, which have been administered to millions of individuals, show us that the vaccines are safe. As we know from prior experience, strain changes can be made without affecting safety," FDA Commissioner Robert Califf tweeted earlier this week.
"When available, new boosters are expected to help provide greater protection against the currently circulating strains. We encourage all who are eligible to consider a booster," Califf wrote.
Because the vaccines have already been studied and administered in millions of people, and the new boosters use the same foundation but change the targeted variant, the FDA is not requiring the same process for authorization.
Health experts say that the decision not to use time-consuming clinical trials for each new shot is also a strategic move, in an effort to keep vaccines up to date with the rapidly evolving variants -- a process that will likely mimic how the flu vaccine is altered each year.
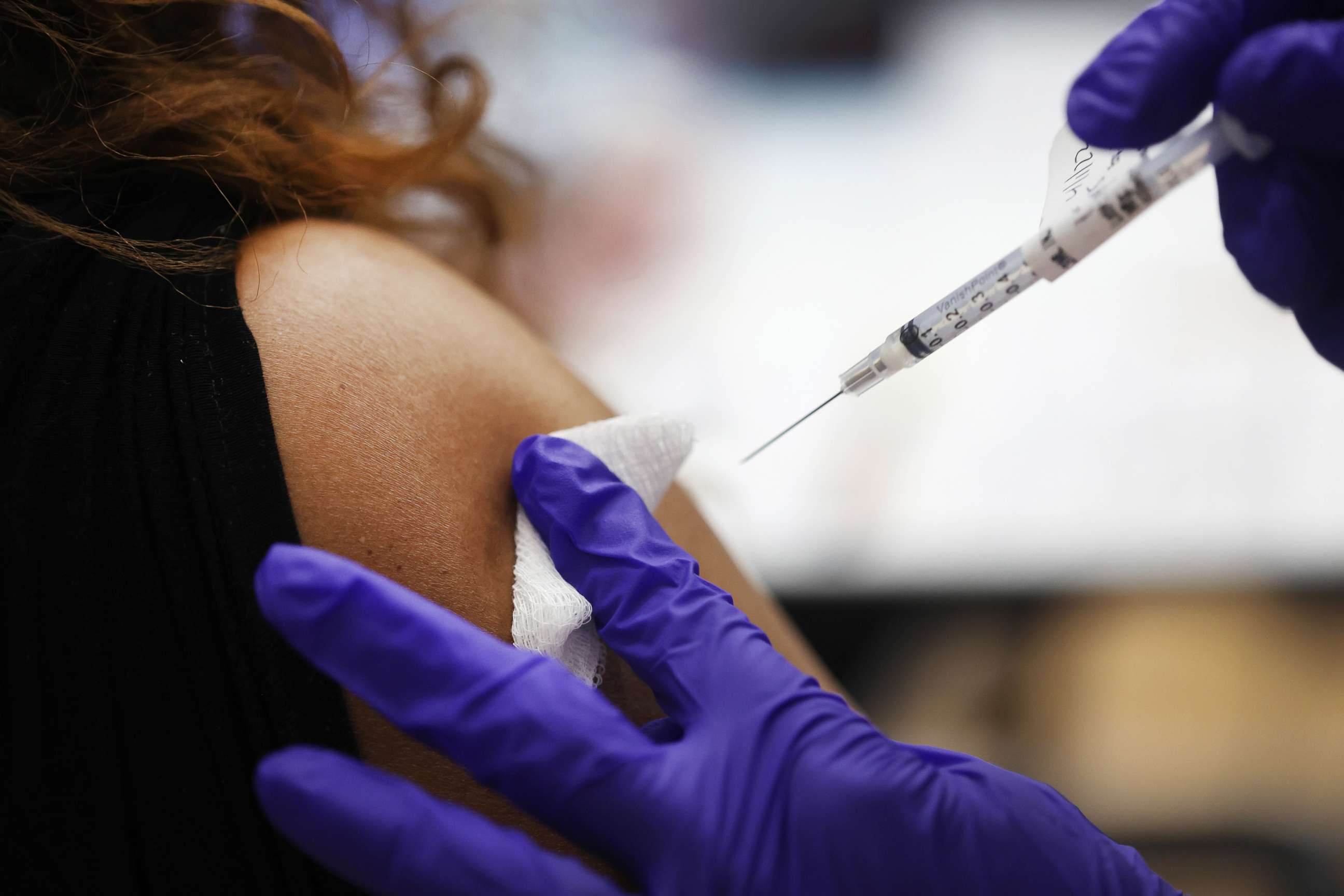
Across the country, 108 million Americans -- or more than half of those eligible to be boosted -- have yet to receive their first booster shot, according to data from the CDC.
Although the immunity provided by COVID-19 vaccines continues to wane with time, data published by the CDC shows that COVID-19 booster doses are still offering protection against severe forms of disease and death, particularly among older Americans.
Among people ages 50 years and older, the unvaccinated had a risk of dying from COVID-19 that was 14 times higher than their fully vaccinated and double-boosted peers.
In people ages 50 years and older, vaccinated people with one booster dose had a risk of dying from COVID-19 that was 3 times higher than those fully vaccinated and double boosted.
More than 61 million people over the age of 50 are eligible to receive their second COVID-19 booster shot, but just a third of people have actually done so. Since second booster doses were authorized in mid-March, a total of 23.1 million Americans have received their second booster.
In May, the CDC announced that it was "strengthening" its recommendation for Americans over the age of 12, who are immunocompromised, and those over the age of 50, to receive their second booster shot.
Younger populations are also benefiting from boosters, data shows.
In June, unvaccinated people ages 12 years and older had a risk of dying from COVID-19 that was eight times higher, compared to people who were fully vaccinated and boosted with their first dose.




