UK regulators say people with a history of serious allergic reactions shouldn't get Pfizer's COVID-19 shot
Authorities in the U.K. were investigating reports of two allergic reactions to the coronavirus vaccine from Pfizer/BioNTech -- a day after the country started its historic inoculation program.
Dr. June Raine, head of the UK regulatory body Medicines and Healthcare products Regulatory Agency (MHRA), said the agency was looking at two reports of allergic reactions to the vaccine, which rolled out yesterday.
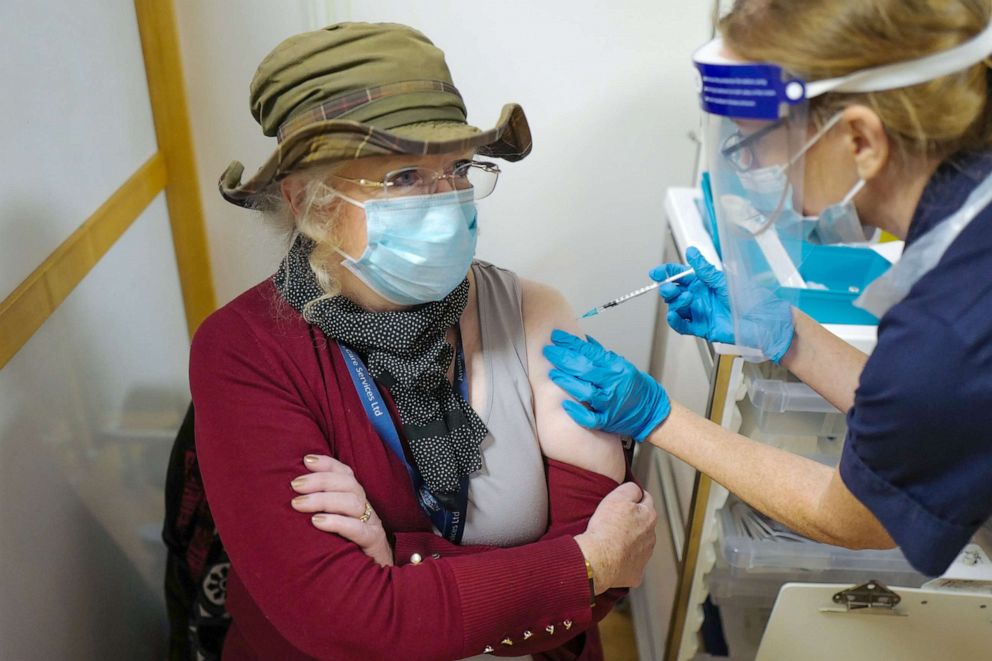
"We know from the very extensive clinical trials that this wasn’t a feature but if we need to strengthen our advice... we get that advice to the field immediately,” said Raine.
The vaccine is believed to be both safe and 95% effective, according to Pfizer/BioNTech. The U.S. Food and Drug Administration was set to hold a hearing for emergency use of the drug on Thursday.
“There have been two cases of anaphylactoid reactions in individuals with a strong past history of allergic reactions both of whom carried an adrenaline auto injector,” the National Health Service (NHS) said in a statement on Wednesday. “These individuals developed symptoms of anaphylactoid reaction shortly after receiving the vaccine. Both recovered after appropriate treatment. We are seeking further information and will issue further advice following investigation.”
Pfizer/BioNTech have not yet commented on these latest findings.
Professor Stephen Powis, national Medical Director for the NHS, said: “As is common with new vaccines the MHRA have advised on a precautionary basis that people with a significant history of allergic reactions do not receive this vaccination after two people with a history of significant allergic reactions responded adversely yesterday. Both are recovering well.”





