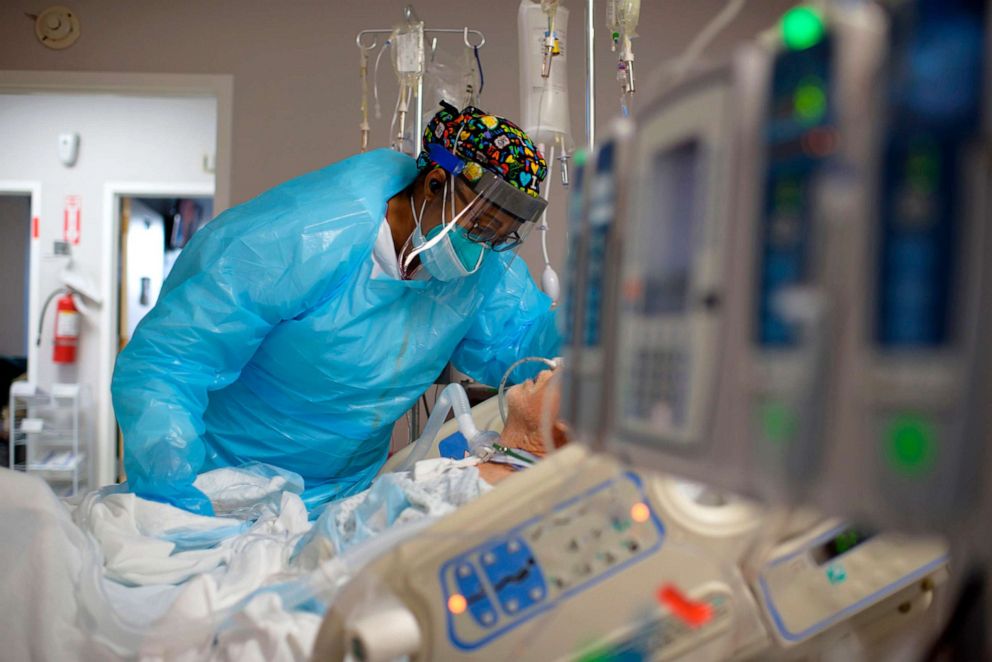
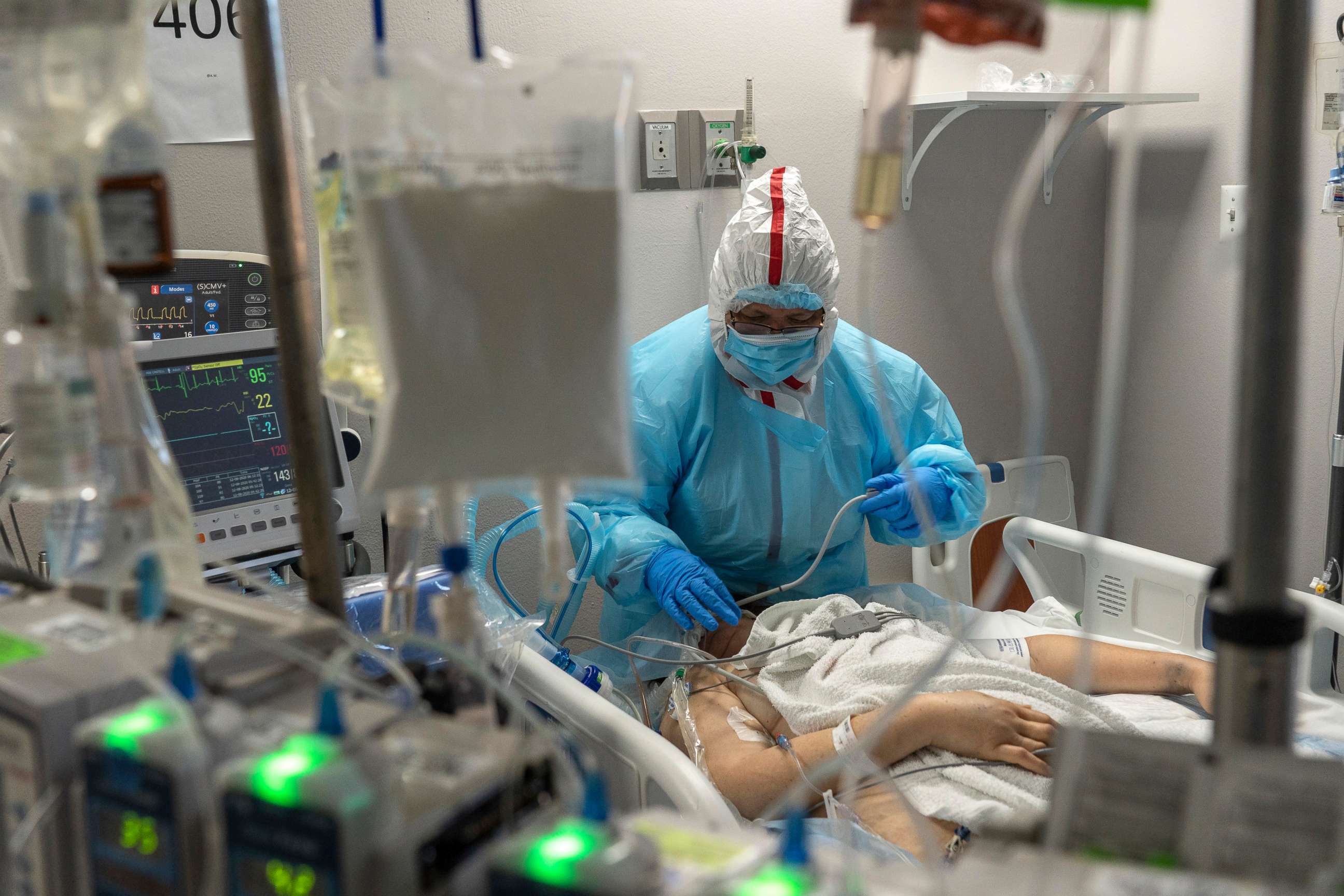
FDA Commissioner: 'We intend to' act quickly on vaccine review
The Vaccines and Related Biological Products Advisory Committee, an independent panel of infectious disease experts, doctors and scientists, is meeting Thursday to recommend if the Pfizer vaccine should be considered safe and effective in the U.S.
Food and Drug Administration Commissioner Stephen Hahn told “Good Morning America” Thursday that he wouldn’t “prejudge” what the advisory committee would vote, but said the FDA will act “quickly” afterward.
“FDA's reviewers are mothers, fathers, brothers, sisters. We totally understand the urgency of this situation, and we are working around the clock on behalf of America,” Hahn said. “FDA scientists are known around the world for their expertise. We are a regulatory gold standard for the authorization or approval of medical products, including vaccines. We intend to do and we have done a very thorough review to get this right, to get all the answers we possibly can from the data.”
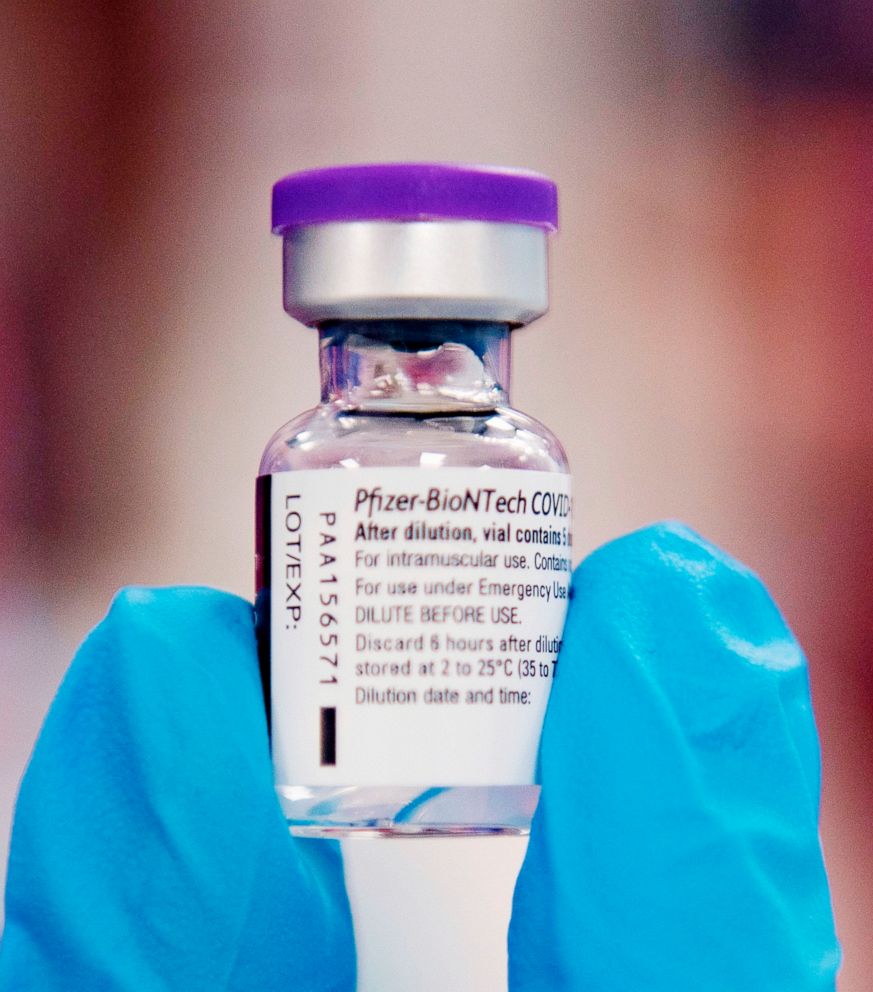
Hahn also said the FDA was “working very closely with our U.K. partners” after two people who received the vaccine in the U.K. had severe allergic reactions.
Hahn told NBC that it was “possible” that the FDA could advise people with significant allergies to not get the vaccine.
Hahn said the allergy issue would be discussed at Thursday’s meeting but added that the FDA stands by “our initial assessment” that Pfizer’s vaccine “does meet our criteria.”
ABC News' Ben Gittleson contributed to this report.