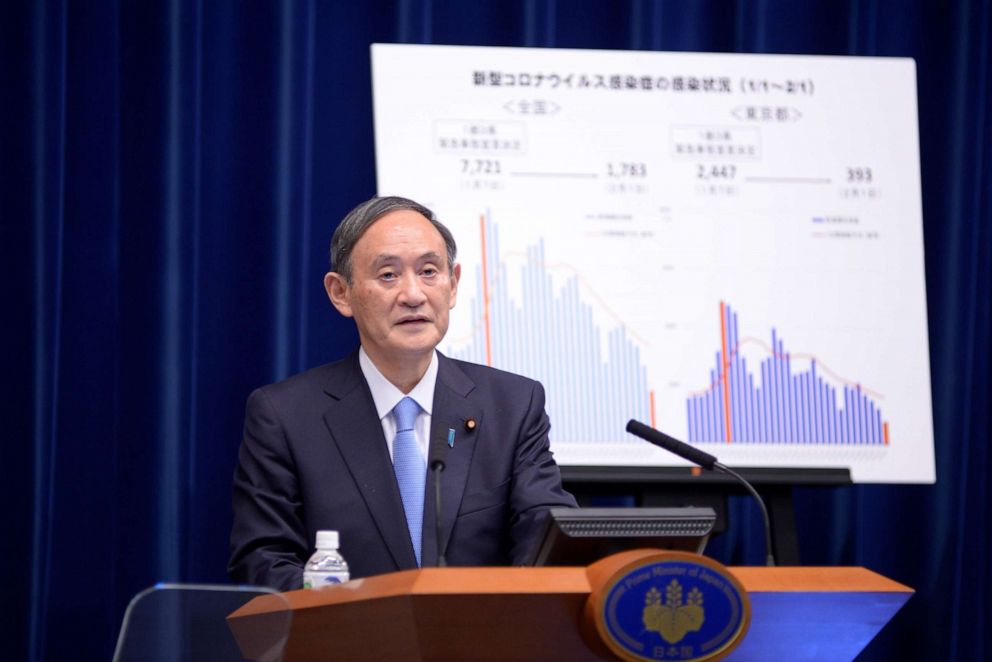
Peer-reviewed study finds Russia's vaccine is over 91% effective
Results from a late-stage clinical trial of Russia's flagship coronavirus vaccine show the shot is not only safe but also 91.6% effective against symptomatic COVID-19, according to a study published Tuesday by the peer-reviewed medical journal The Lancet.
The randomized, double-blinded, placebo-controlled Phase 3 trial in Moscow involved 19,866 adult participants, of whom 14,964 received the vaccine, called Sputnik V, and 4,902 received a placebo. The two-dose vaccine was administered 21 days apart.
There were 62 confirmed cases of COVID-19 identified among the trial participants in the placebo group and 16 cases in the vaccine group, according to the study.
The study said there were no serious adverse effects related to the vaccine recorded during the trial and that 94% were mild, including flu-like symptoms, pain at the injection site and headaches.
A sub-analysis of 2,144 trial participants older than 60 showed the vaccine had a similar efficacy of 91.8%, according to the study.
"Our interim analysis of this phase 3 trial of Gam-COVID-Vac has shown promising results," the researchers wrote.
The publication of peer-reviewed data follows last year's criticism of Russia for registering the COVID-19 vaccine -- and declaring itself the first country in the world to do so -- before starting crucial Phase 3 trials.
"The development of the Sputnik V vaccine has been criticised for unseemly haste, corner cutting, and an absence of transparency," Ian Jones, a professor of virology at England's University of Reading, wrote in a comment piece published Tuesday by The Lancet alongside the study. "But the outcome reported here is clear and the scientific principle of vaccination is demonstrated, which means another vaccine can now join the fight to reduce the incidence of COVID-19."
Russia’s Direct Investment Fund (RDF), which funded the production of Sputnik V and is responsible for its worldwide marketing, hailed the study and noted that the COVID-19 vaccine is currently one of only three in the world with an efficacy above 90%. The vaccine, which is now registered in 16 countries, costs $10 per dose and can be stored and transported more easily because it can be kept at the temperature of a standard refrigerator, according to RDF.
"The data published by The Lancet proves that not only Sputnik V is the world’s first registered vaccine, but also one of the best," Kirill Dmitriev, CEO of RDF, said in a statement Tuesday.
ABC News' Patrick Reevell contributed to this report.