The race for a COVID-19 vaccine: Fast, but fast enough?
A vaccine within 12 to 18 months is "probably not going to happen."
Dozens of scientists across the globe are expeditiously developing and testing a variety of therapeutic treatments for COVID-19, the respiratory illness caused by a novel coronavirus. Meanwhile, dozens more are working to protect those who have not yet been affected by creating a vaccine.
The Department of Health and Human Services just announced that the government is working with major pharmaceutical and biotech companies to speed up the development of COVID-19 vaccine trials and, ultimately, the manufacturing of said vaccines.
As Dr. Anthony Fauci, the head of the National Institute of Allergy and Infectious Diseases, keeps reminding us, the development timeline for treatments and vaccines are "fundamentally different."
Although it likely will take more than a year before a vaccine is ready for the public, Fauci also said COVID-19 vaccine development is on track to be "literally the fastest that we have ever done."
Tune into ABC at 1 p.m. ET and ABC News Live at 4 p.m. ET every weekday for special coverage of the novel coronavirus with the full ABC News team, including the latest news, context and analysis.
With more people being infected and lockdown orders creating immense financial burdens with so many not allowed to work, the question becomes even more pressing: Is there a viable vaccine in sight?
Dr. Maria Elena Bottazzi, co-director of the Texas Children's Hospital Center for Vaccine Development, believes a previously stated 12- to 18-month timeline may be too optimistic.
"My concern is that people are hearing we are going to see things in a year or 18 months," Bottazzi told ABC News. "The reality is: That's probably not going to happen. We may have some real optimism and some safety evidence by then, but not a vaccine."
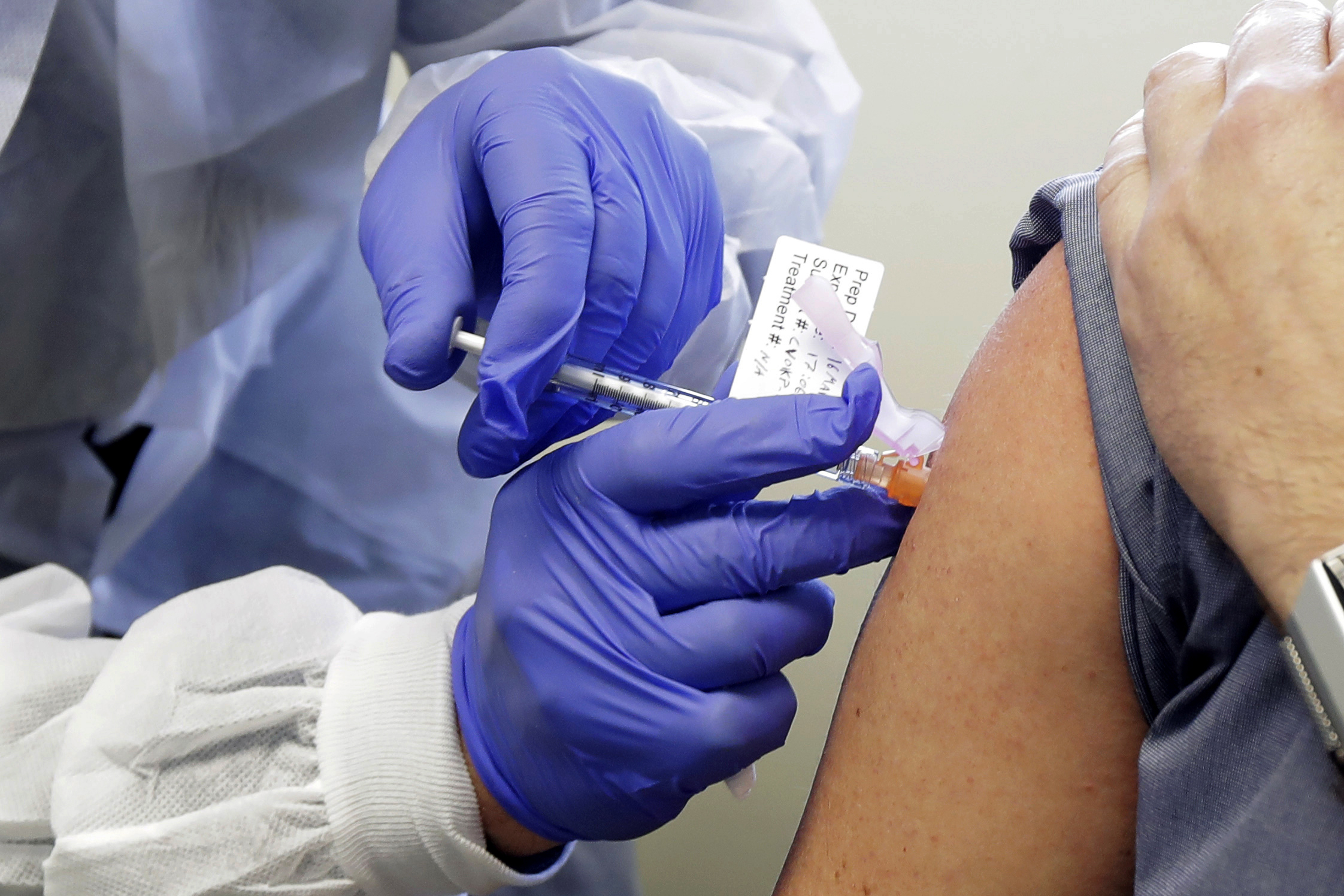
"There's a long road after safety -- you still have to do a lot of things before you can confirm the efficacy and give this to the public en masse," Bottazzi added. "That's the hard message. It's not just a 30- or 40-people trial to lead us to a solution."
The long road to vaccine development
Vaccines train a person's immune system to recognize a pathogen and attack it before it infects healthy cells. Vaccines can prime the immune system, give it a head start, get it ready to fight.
But before vaccines can be tested for efficacy, they undergo even more safety testing than ordinary medicines. As Bottazzi noted, vaccines are intended for use among healthy people and there's "a high bar to make sure they are going to do more good than harm."
Safety is the focus of Phase I testing, while Phase II involves determining whether or not the vaccines work. Bottazzi noted that many of the front-runner vaccine candidates that have received both media attention and critical funding dollars are new and unproven, and some use technology never used in humans.
"The vaccines that have been funded and accelerated are all novel technologies -- I'm not saying that they are not going to work, but they are novel," Bottazzi cautioned.
Some candidates, such as a vaccine by Cambridge, Massachusetts-based biotechnology company Moderna, use a technique never before been used on humans. Despite relying on new technology, Moderna's vaccine was rushed to Phase I trials before it was tested on animals, which, Bottazzi said "is very unusual."
Mass producing vaccines before they're proven
In a White House briefing last week, Fauci pointed out that vaccine production, too, would need to be expedited -- likely at an unusual stage of development.
Typically, pharmaceutical companies would wait to see if their vaccine works in clinical trials before ramping up production. Not so with this pandemic, because, experts have said, because production on large quantities will need to begin before having complete data. That way, they can be ready to distribute widely when it's finally proven safe and effective.
"Even before you know something works," Fauci said, "you have to start producing it. Because once you know it works, you can't say, 'Great it works -- now give me another six months to produce it.'"
Those tactics present a risk, Fauci acknowledged, because it's unclear which vaccines now being tested will end up working, or working the best, but it's a risk developers must take. There's also concern using new technologies may create additional challenges.
"Vaccines should be easy to make -- generic enough that everyone can reproduce them, with minimum proprietary technology," Bottazzi said, adding that doing so can prevent "another big bottleneck for vaccines."
Her group at Texas Children's Hospital and other vaccine producers are staying with tried-and-true development methods, including the incorporation of a complimentary ingredient called an "adjuvant," which boosts the immune system's response to the vaccine.
"Our vaccine uses the simplest and safest adjuvant possible: aluminum, the only adjuvant that has been licensed all over the world," she said.
Not all vaccines, including several COVID-19 front-runners, use adjuvants, but many researchers consider it safer. Compounds including adjuvants require less of the new drug, also called the "antigen," which reduces potential side effects and makes the overall vaccines cheaper to produce.
"Adjuvants classically will allow you to get a robust immune response at a tenfold-lower antigen dose," said Dr. Ofer Levy, an infectious disease physician and leader of the Precision Vaccines Program at Boston Children's Hospital. "How are you going to scale a vaccine to a billion doses without an adjuvant? I'm not seeing it."
Echoing that newer vaccine technologies may be more expensive, time-intensive and difficult to mass produce, Levy said his group at Harvard instead is focusing on finding an adjuvant that's particularly effective in vulnerable populations.
In the meantime, experts are hopeful another type of therapy soon will be available: convalescent plasma, a still-experimental treatment in which antibodies from someone who recovered from COVID-19 are shared via infusion. This method could be used to treat those hospitalized with the novel coronavirus as well as boost the immune systems of healthy people at the greatest risk of contracting it.
Patients at New York's Mount Sinai Hospital now will be administered convalescent plasma under careful supervision.
Bottazzi said: "Bottom Line: Let's continue to be optimistic, but at the same time be aware that it will take a lot of time, trial and error, and failures."
What to know about coronavirus:
- How it started and how to protect yourself: Coronavirus explained
- What to do if you have symptoms: Coronavirus symptoms
- Tracking the spread in the U.S. and worldwide: Coronavirus map
Chloë Nunneley, M.D., a resident physician in pediatrics at Boston Children's Hospital and Boston Medical Center, is a contributor to the ABC News Medical Unit.





