FDA warns: Don’t let your children sink their teeth into benzocaine
The FDA says benzocaine-based products should no longer be used for teething.
The crying echoes through your house – your teething baby is miserable, and all you are wishing for is some peace for the both of you.
Here’s what a new FDA warning says a parent should NOT do.
Don’t run to the nearest drug store and buy all of the products made for the relief of sore gums – these products could be a problem, according to the new warning.
Many over the counter products designed for teething pain contain benzocaine, a chemical that may pose serious health risks for infants and children. The FDA says these products should no longer be used on children under 2.
Benzocaine is associated with a dangerous health condition called methemoglobinemia, which causes the amount of oxygen carried through the blood to be greatly reduced. It can be fatal.
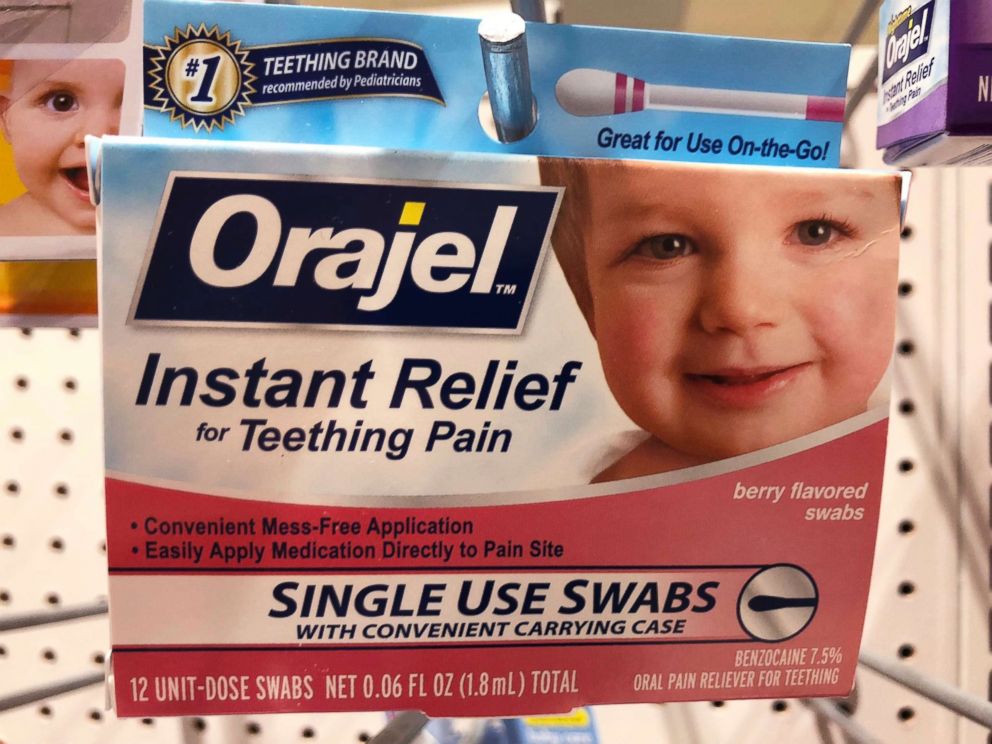
FDA officials are requesting that these products no longer be marketed or sold – or, at the least, that companies add warnings with up to date drug safety information to all oral health products containing benzocaine.
If companies do not comply, FDA officials warn that they could take regulatory action to remove these products from the market.
Here’s what parents should do.
Check your teething relief product labels to see if benzocaine is an active ingredient. If products with this chemical are on your shelves, toss them.
If you use a teething remedy and your child becomes pale, or looks gray or blue in their lips and nail beds, has shortness of breath, fatigue, headache, lightheadedness, and rapid heart rate, they need immediate medical attention.
These new directives may have parents wondering - what are they supposed to do with a teething baby?
The traditional recommendation of a gentle massage with your fingers on your child’s gums is the go-to treatment.
Eric M. Ascher, DO, is a third-year family medicine resident from New York working in the ABC News Medical Unit.




