FDA authorizes Pfizer's booster shot for 12- to-15-year-olds
The U.S. Food and Drug Administration authorized booster shots of Pfizer-BioNTech's COVID-19 vaccine in children between ages 12 and 15 on Monday.
Booster shots have been touted as a key tool in fighting the surge in COVID cases linked to the omicron variant, which has shown an ability to -- at least partially -- evade protection offered by two doses.
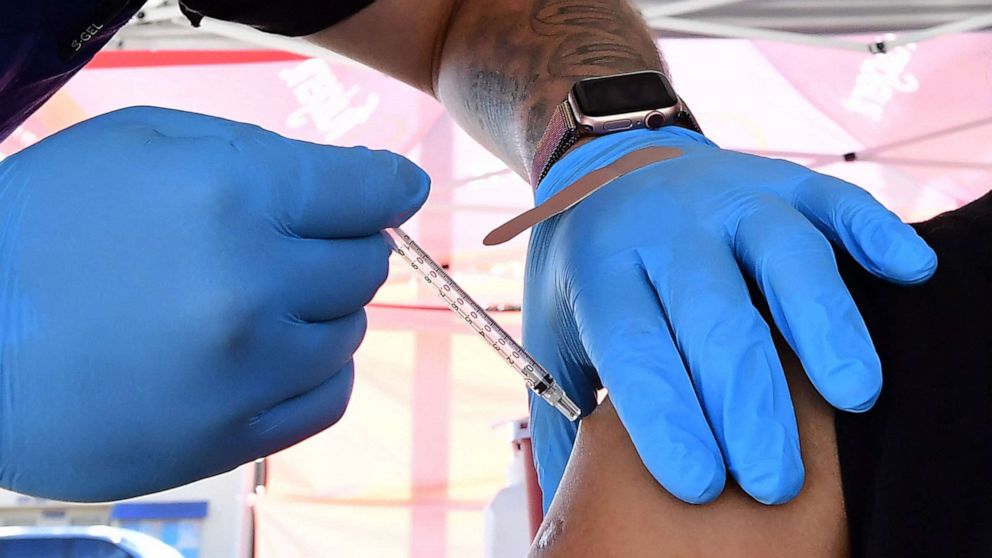
The FDA also shortened the wait period for adults and adolescents to receive boosters from six months down to five months.
In addition, the agency authorized COVID booster shots for children aged five to 11 who are immunocompromised.





