What to know about monkeypox vaccination that could stretch supplies
Intradermal vaccination can save supplies and protect more people.
Amid a newly announced monkeypox national public emergency and shortage of vaccines, the Food and Drug Administration announced it is reviewing a new vaccine approach that could lead to a fivefold increase in the US’s supply of the Jynneos monkeypox vaccine.
“Please know we've been exploring all scientifically feasible options, and we believe this could be a promising approach,” said FDA Commissioner Dr. Robert Califf, speaking during a Thursday press briefing.
The vaccine would be given in a smaller, shallower injection under the skin, a method Califf said would still be safe, effective, and would allow up to five doses to be pulled from one vial.
The new strategy will still need to be tested in clinical trials -- a process that could take weeks or months. But experts say prior studies look promising, and if successful, this could be a safe way to stretch limited vaccine supply.
"This kind of research is exactly what FDA and NIH should be leading in this moment of public health emergency," said Dr. Josh Sharfstein, a former FDA Commissioner and currently vice dean for public health practice and community engagement and director of the Bloomberg American Health Initiative at Johns Hopkins Bloomberg School of Public Health.
"If much less vaccine can be used with intradermal injection, as opposed to intramuscular injection, then the current monkeypox vaccine supply can be stretched to accommodate many more people."
The Jynneos monkeypox vaccine is currently FDA approved as a subcutaneous shot that’s given in two doses four weeks apart. The new strategy would still be a shot given in two doses, according to the CDC.
Stretching the supplies could help “close the gap,” Califf said. The White House has already made 1.1 million doses available to states and expects 6.9 million doses to be delivered by May 2023, according to the HHS.
Here's what to know about the new process:
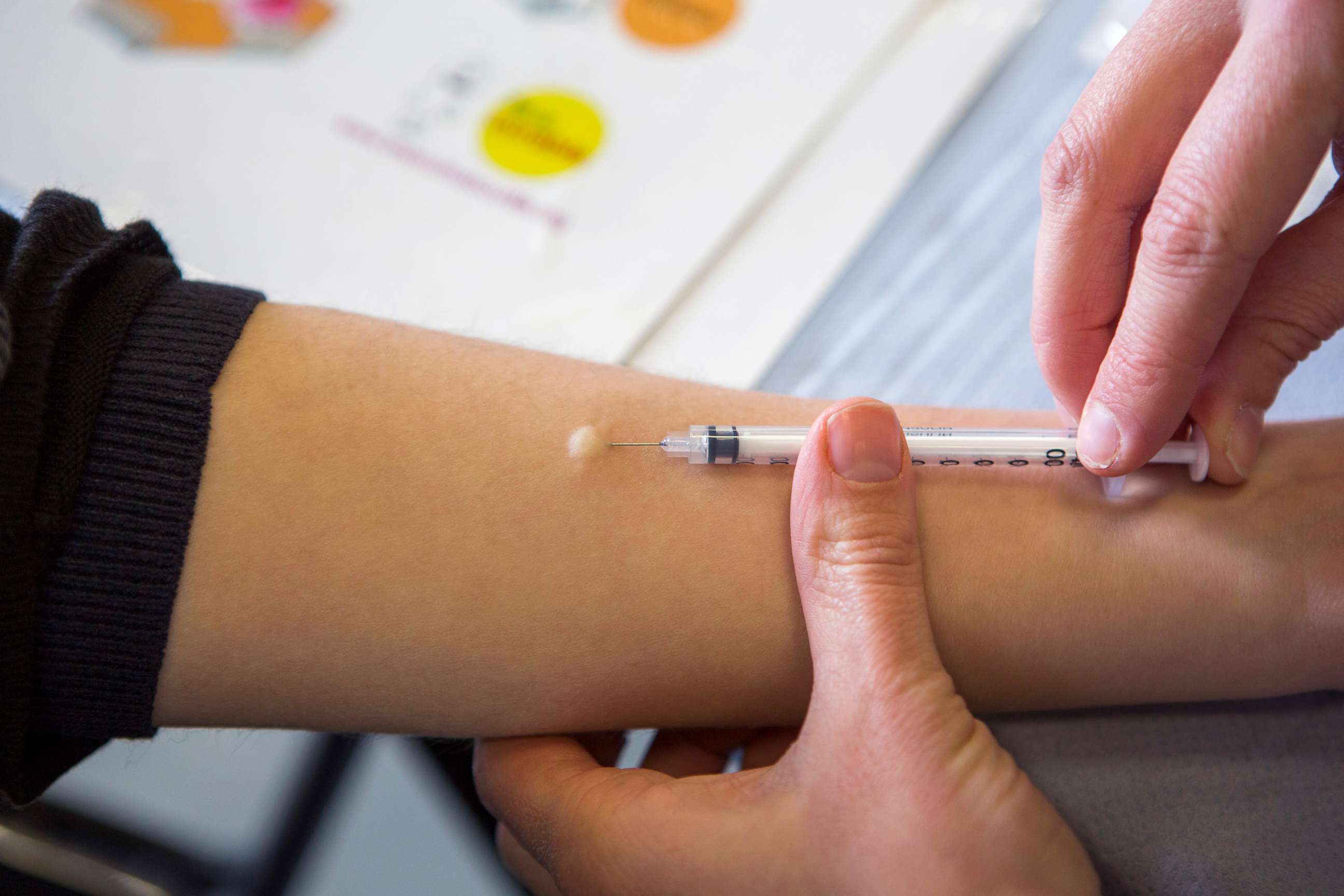
What is an intradermal injection?
The dermis is the second layer of the skin, below the epidermis, which is the visible layer of skin. Most routine vaccinations are given as intramuscular or subcutaneous shots that go deeper than the dermal layer.
But a vaccine given intradermally would make a small bubble under the skin. This is the exact same technique already used in tuberculin skin tests also called a PPD test.
How will the FDA know if this works?
This smaller dose will need to be tested like any other vaccine to make sure it is both safe and effective.
“It's important to note that overall safety and efficacy profile will not be sacrificed for this approach,” Califf said.
But this isn’t like starting a vaccine from scratch and other vaccines have been shown to work using this method. It was used around the world against smallpox in the 1970s.
“Smallpox vaccine that was used globally to eradicate smallpox from the planet required intradermal vaccination,” Dr. Richard Kuhn, Krenicki Family Director of Inflammation, Immunology, and Infectious Disease at Purdue University and Editor in Chief of Virology told ABC News.
Dr. Dan Barouch, the William Bosworth Castle Professor of Medicine Harvard Medical School, Ragon Institute of MGH, MIT, and Harvard and Director, Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center, says the intradermal method, “is very effective because there are a lot of immune cells just under the skin, and often lower doses are needed compared with intramuscular vaccination.”
More recently, the influenza vaccine against the H1N1 strain that caused a pandemic in 2009 has been tested intradermally in many research studies and was shown to be just as effective at one-fifth the dose of the intramuscular shot.
How will the shots get into arms?
If the Jynneos monkeypox vaccine passes a clinical trial, there is another barrier that will need to be overcome. Barouch also warns that administering a vaccine this way may pose a technical challenge, saying “most people are not trained or experienced in administering vaccines by the intradermal route.”
But experts say it’s a skill that can easily be learned.
“Whomever could be expert in intramuscular administration, but you still would want to give them the training for the intradermal. Like I said, they could be taught very quickly, and they could be off and running in a very short period of time,” Dr. Robert Frenck, director of the Vaccine Research Center at Cincinnati Children’s Hospital Medical Center explains to ABC News.
Frenck also says this national public health emergency is a reminder that living in a globalized world means an outbreak anywhere is a danger to people everywhere.
"That's why the vaccines are so important. That's what keeps us healthy," Frenck said.
Dr. Jade A Cobern, board-eligible in pediatrics, is a member of the ABC News Medical Unit and a general preventive medicine resident at Johns Hopkins.
Cheyenne Haslett contributed to this report.




