How to prepare for the coming wave of COVID child vaccinations: OPINION
Agencies should use targeted approaches to vaccinate vulnerable children.
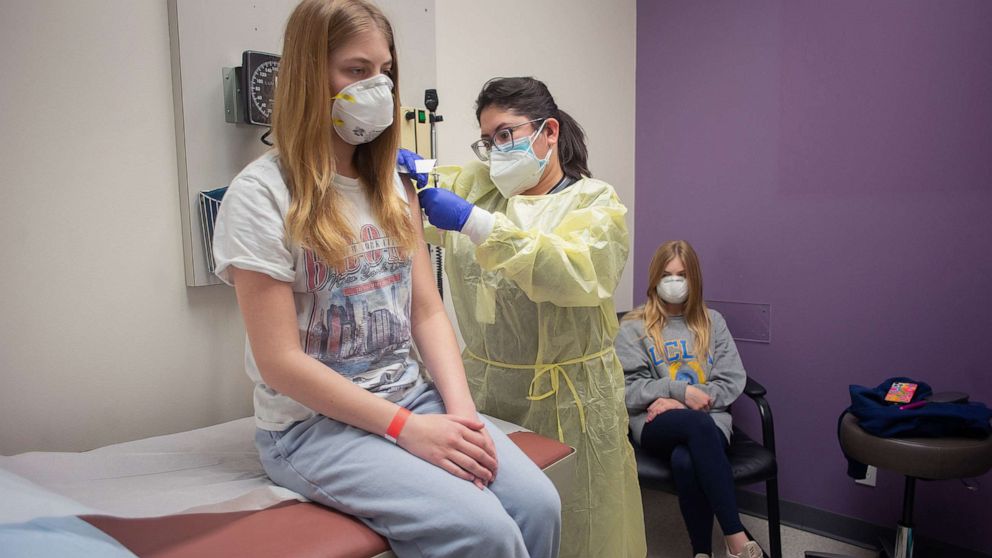
While there’s a high level of certainty that we will have a vaccine ready for some children later this May, how we will distribute the vaccine effectively and equitably to this group remains unclear.
We are waiting on the Food and Drug Administration's review of the data submitted on the Pfizer-BioNTech COVID-19 vaccine for 12 to 15-year-olds. If and when the likely emergency use authorization is granted, the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices (ACIP) will meet the following day to make recommendations, which would apply to over 20 million kids. These kids would likely become eligible for the vaccine just as the school year is ending and summer plans await.
Pfizer-BioNTech and Moderna are also conducting trials of their mRNA vaccines in children ages 6 months through 11 years, and this data will likely be submitted in the fall of 2021.
While we have incomplete data from only 24 states and New York City on pediatric hospitalizations due to COVID-19, we know that numbers are on the rise. The American Academy of Pediatrics reported that children represented 22.4% of new cases reported the week of April 22, up from one year ago when children comprised only 3% of total U.S. cases. We are also tracking kids who have experienced a serious complication of COVID-19, known as a multisystem inflammatory syndrome in children.

New studies are in progress to understand the effects of long-haul COVID-19 in kids and families. There is concerning data that Black and Latinx kids are more likely to die of COVID, and their parents continue to worry about school safety. We know that COVID-19 is a vaccine-preventable disease. Vaccinating children will facilitate in-person schooling, which is essential for short and long-term well-being.
We grapple with the ethics of vaccinating adolescents ahead of high-risk adults in other countries. The U.S. has a dual role to play while improving vaccine access at home; we must also commit to expediting global supply.
Some of the lessons we learned from past vaccination rollouts can also inform safe and rapid COVID-19 vaccine allocation and distribution among children. Polling data have also shown that demand for COVID-19 vaccination is dynamic and a significant driver of how many people get vaccinated.
We already see states and jurisdictions preparing and convening pediatric vaccine planning teams. Washington's vaccine planning team includes representatives from the District’s chapter of the American Academy of Pediatrics (AAP), pediatric providers, public schools, Families USA and March of Dimes. North Carolina plans to partner with schools to help identify children for vaccination and is engaged with its AAP and the Pediatric Society to support vaccine education and communication.
In addition, policy changes have expanded our pool of pediatric vaccinators. Previously, the minimum age of a child a pharmacist could immunize depended on state regulations, and only 28 states permitted pharmacists to vaccinate children in general. On Aug. 24, 2020, the U.S. Department of Health and Human Services began to allow pharmacists in all states to undergo additional training to administer vaccines to children as young as 3 years of age.
A Kaiser Family Foundation survey found that willingness of parents to immunize their children in the 12-15 age group was only 30%, with a quarter of parents waiting to see how the vaccine is working. Nearly another quarter was unwilling to vaccinate their children.
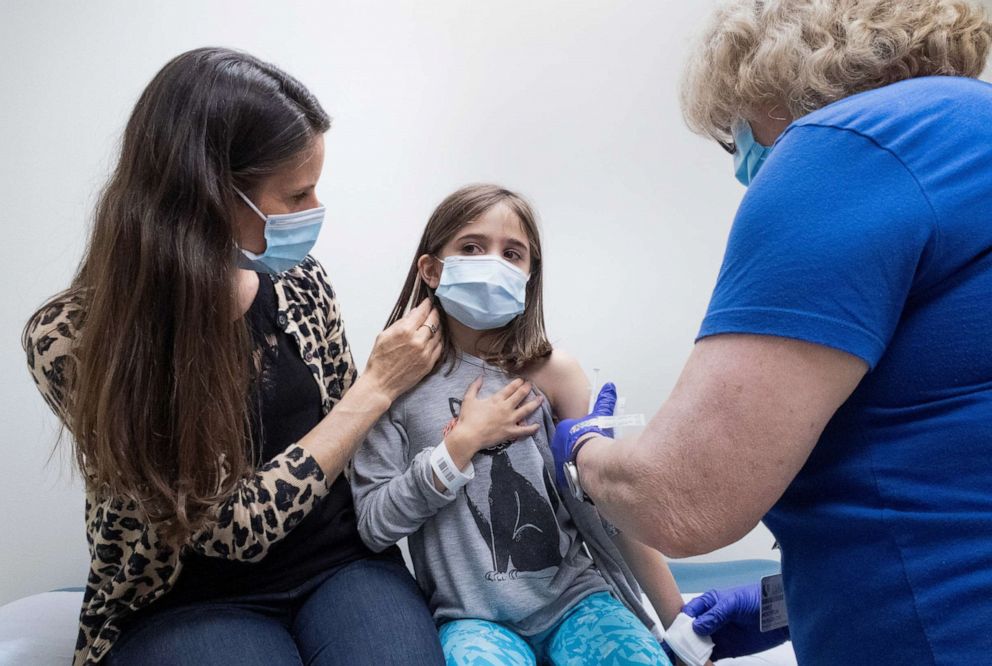
Given the likely imminent authorization of vaccine for adolescents 12-15 years of age, we must expedite planning now, extending work in three key areas:
#1 Allocation: In the first phase, federal and state agencies should use targeted approaches first to vaccinate vulnerable children, immunocompromised children and children who live with immunocompromised adults.
#2 Distribution: State and federal planners should adopt a uniform Social Vulnerability Index to allocate vaccines to communities hardest hit by the pandemic’s economic, health and social conditions. Authorities should map and detail which areas have access and mitigate vaccine deserts. Offering resources and infrastructure for school-based clinics appears to boost vaccine uptake is another effective strategy.
Schools can become effective vaccine hubs; previous rollouts have demonstrated their success in providing vaccines, especially among economically disadvantaged and medically underserved areas. During the 2009 H1N1 pandemic, schools were identified as being primary sites for administration of vaccines to children, staff and the school community. According to the National Association of School Nurses, school vaccination events have a long history in the United States. They have successfully contributed to lower morbidity and mortality due to vaccine-preventable diseases.

#3 Vaccine Confidence: Improving access improves demand. Parents are the gatekeepers and will respond to guidance from their primary care providers and pediatricians. Pediatricians and their staff will have to be proactive on outreach and coordinating schedules if vaccines have been missed. We need to have as many school vaccine days until the end of the school year. This will require a scheduling system that allows families to schedule the shots together and offer walk-in and signups for all family members over the age of 12.
Workplaces can offer incentives for employees, including paid time off, family vaccine days and other creative ways to establish vaccination as a positive way to mark the next chapter. We need to see families as the unit and build systems to support families navigating the web of providers and schedules.
This is not a childhood immunization campaign for a disease that we rarely see. The vaccine rollout continues to be a central strategy to protect the vulnerable, mitigate transmission and end the pandemic. Now is the time to invest in the scheduling, communication and workforce that create a positive vaccination experience for kids. We have a narrow window to boost confidence and uptake of the COVID-19 vaccine and minimize risks for kids and their families.
Rebecca Weintraub, MD, is an assistant professor at Harvard Medical School and associate physician at Brigham and Women's Hospital.
Asaf Bitton, MD, MPH, is executive director at Ariadne Labs, a joint center for health systems innovation at Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health.
John Brownstein, Ph.D, is an ABC News contributor. He's also the chief innovation officer at Boston Children’s Hospital and a professor at Harvard Medical School.




