3 siblings get Pfizer-BioNTech COVID-19 vaccine as part of global trials in young children
The children's parents discussed their decision with ABC News.
Three American siblings have received their first dose of the Pfizer-BioNTech COVID-19 vaccine as part of global clinical trials for young children.
Christian Bui, 3, and Sloan Bui, 14 months, both got the initial shot on Monday at Ochsner Hospital for Children in Jefferson, Louisiana, just outside New Orleans. Their older sister, 6-year-old Ellie Bui, got her first dose earlier this month.
The children's parents, Dr. Cuong "CJ" Bui and Dr. Erin Biro, are neurosurgeons at Ochsner Medical Center in Jefferson and discussed with ABC News their decision to have all their kids participate in the study.
"For us, our kids living safely in a world where we don't have to worry about them getting sick from COVID, being able to go to school, have playdates with their friends, we feel strongly that vaccine is what is going to get us to those goals," Biro said.

The mother also expressed worry over the so-called delta variant, a highly contagious strain of the novel coronavirus first identified in India that has since been detected in more than 80 countries around the world and in at least 41 U.S. states, including Louisiana.
"The delta variant has really picked up steam in the U.S. and I think in Louisiana especially, given the fact that so much of our population is not vaccinated yet that it has a really significant chance of causing an uptick in COVID cases in Louisiana," Biro told ABC News. "So we feel fortunate that we have the possible chance of having our kids protected, but more so getting the pediatric trial across the finish line so that all kids have the possibility of being protected from the variant if it becomes more significant or if there's more cases this fall."
"We're super excited that our entire family now has the opportunity and the chance of possibly being protected," she added, "and also just contributing to the research and the science to hopefully get all kids across the finish line."
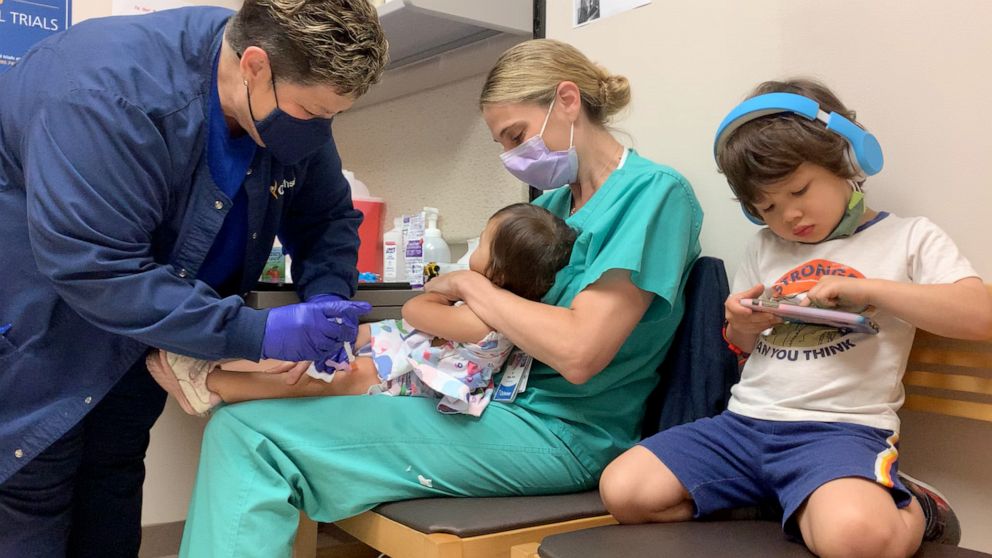
In November, American pharmaceutical giant Pfizer and its German partner BioNTech began enrolling child volunteers aged 12 to 17 in the Phase 2/3 trials of their two-dose COVID-19 vaccine. Now, volunteers as young as 6 months are being enrolled. The placebo-controlled study will include up to 4,500 participants from the United States, Spain, Poland and Finland, according to Pfizer.
Ochsner Hospital for Children, one of two locations in Louisiana to offer the trial, announced earlier this month its participation as a site for the global study and said it is enrolling as many as 75 participants.
"Now that the vaccine has proven to be effective in adults, it is a natural next step in the research process to study the unique needs of younger individuals," Dr. Julia Garcia-Diaz, Ochsner's director of clinical infectious diseases research, said in a statement. "A child’s immune system is different than an adult’s immune system, so it is critically important to have a study focused on the efficacy of the vaccine in this cohort."
In December, the U.S Food and Drug Administration granted emergency use authorization for the Pfizer-BioNTech vaccine in people aged 16 and up, after data showed the shot was safe and 95% effective in a massive Phase 3 trial. The FDA then expanded the temporary authorization in May to include individuals as young as 12, after data from a Phase 3 trial showed the vaccine was safe and 100% effective in adolescents aged 12 to 15. For younger age groups, Pfizer and BioNTech would potentially apply for emergency use authorization of the vaccine in the U.S. shortly after getting trial results, assuming the data shows the vaccine to be both safe and effective.
Pfizer and BioNTech have already applied for full FDA approval of the vaccine for use in people 16 and older.
ABC News' Erica Baumgart, Arielle Mitropoulos, Marcus Moore and Jim Scholz contributed to this report.




