CDC director overrules panel on Pfizer boosters for front-line workers
The shots will become available for millions more Americans as soon as Friday.
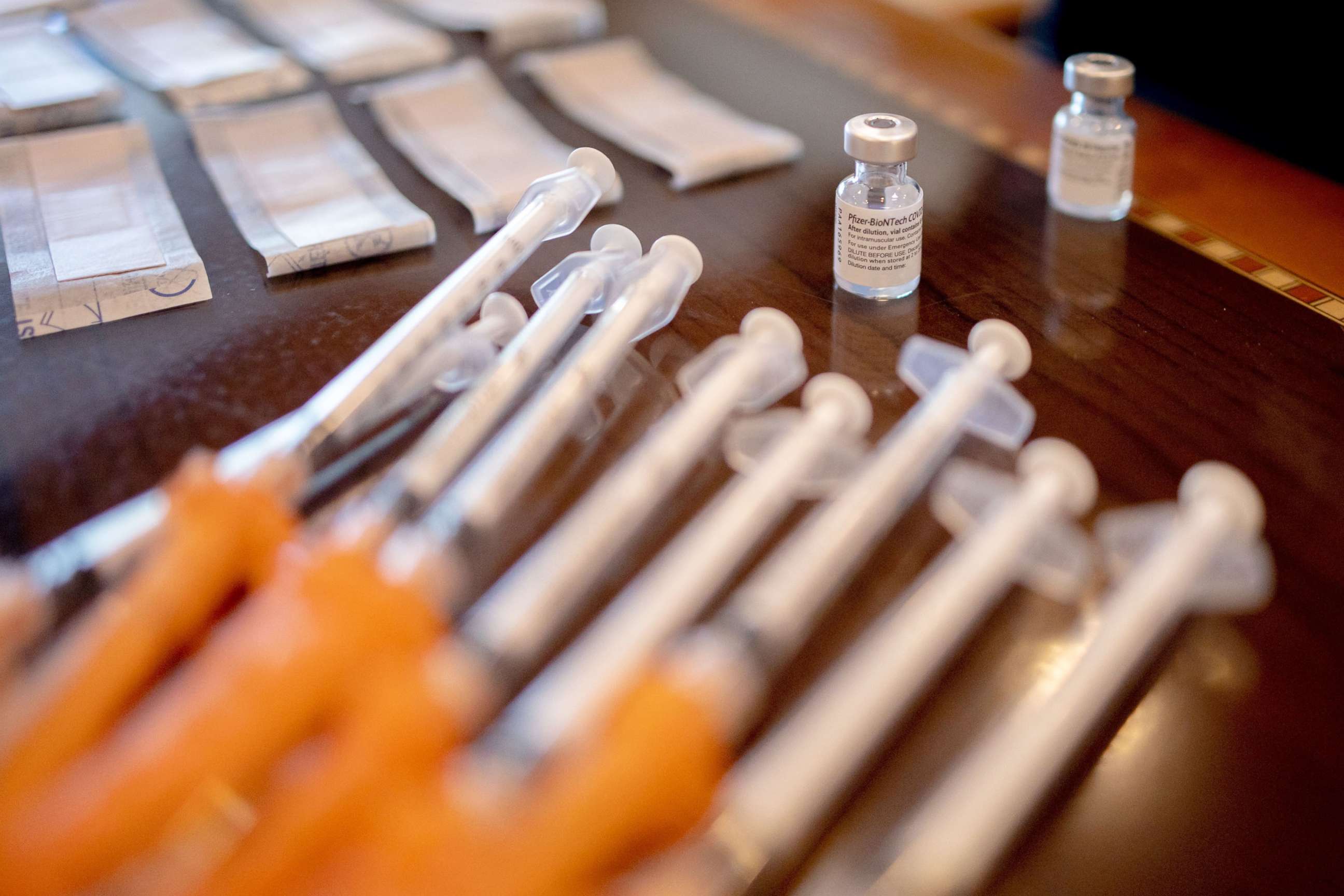
The Centers for Disease Control and Prevention has endorsed an independent advisory panel's recommendation for seniors and other medically vulnerable Americans to get a booster shot of Pfizer's COVID-19 vaccine, six months after their second dose.
Dr. Rochelle Walensky, director of the CDC, also partially overruled her agency's advisory panel in a notable departure by adding a recommendation for a third dose for people who are considered high risk due to where they work, such as nurses and teachers -- a group which the panel rejected in its recommendation. Some panelists said that without further data, they weren't comfortable with automatically including younger people because of their jobs.
In a statement announcing her decision late Thursday, Walensky pointed to the benefit versus risk analysis she had weighed, and data rapidly evolving.
"In a pandemic, even with uncertainty, we must take actions that we anticipate will do the greatest good," Walensky said. "While today’s action was an initial step related to booster shots, it will not distract from our most important focus of primary vaccination in the United States and around the world."
With Walensky's final sign-off, booster shots will now quickly become available for millions more Americans at pharmacies, doctors' offices and other sites that offer the Pfizer vaccine as soon as Friday.
The CDC's independent advisory panel voted unanimously on Thursday to recommend Pfizer boosters for people aged 65 and older, along with long-term care facility residents and people as young as 18, if they have an underlying medical condition.
People younger than 49, however, should only get a third dose if the benefits outweigh the risks, the panel said -- a personal consideration to discuss with their doctor.

Walensky's endorsement at least in part buttons up what has become a seething scientific debate after the Biden administration announced "boosters-for-all" ahead of any reviews from the regulatory bodies, or their independent groups. While the White House's political appointees had endorsed Biden's timeline, some of their career scientists and advisers vehemently objected to the incomplete data they were being asked to assess.
Ahead of Thursday's vote, Walensky addressed the panelists and thanked them for "leaning in" to the complex issue at hand and "trying to put the pieces together."
"You're tasked with difficult decisions, weighing the risks and benefits extrapolating from sometimes a wealth and sometimes a paucity of data available," Walensky said, but reminded them that despite the complex and contentious debate they share the goal of pulling the nation out of the pandemic.
"We all recognize that the science and data of COVID-19 are moving faster than any data we've ever seen before. And while I recognize a tremendously heavy lift of the past year, we all know that the pace is unlikely to let up anytime soon," she added. "We will continue this dialogue, you will have more data to review and more recommendations to make and I will be here with you."
Not every panelist was excited about the idea of boosters, insisting the vaccines still provide remarkable protection and that it was unvaccinated Americans who remained most at risk.
"I feel like we're putting lipstick on hogs. This is not going to solve the pandemic," said Dr. Keipp Talbot, a voting panel member and infectious diseases professor at Vanderbilt University in Nashville, Tennessee.
The panel's vote narrowed Wednesday's authorization from the Food and Drug Administration, which did agree to make the shots available to front-line workers.
The vote also followed weeks of a contentious back and forth among top health experts over who should get a booster dose and when -- and whether it's still premature to be asking the question.
Scientists agreed that while vaccine protection is waning slightly, on the whole, vaccines are still working to dramatically reduce the risk of hospitalization. And many feared endorsing booster doses for most would imply vaccines are no longer working.
"I feel that we're getting too much ahead of ourselves and that we have too much hope on the line with these boosters," said voting member Dr. James Loehr of Cayuga Family Medicine in Ithaca, New York. "Having said that, you shouldn't let the perfect be in the way of the good."
Panelists initially pushed back on the proposals that American adults, 18 to 64, who are at risk for severe COVID-19 infection due to underlying medical conditions, or due to their occupation and setting receive a Pfizer booster dose. Many members stressed that in order to truly "move the dial" on the pandemic, more people need to complete the initial vaccination series.
"I think two and three are fraught with peril," said member Dr. Oliver Brooks, chief medical officer of Watts HealthCare Corporation in Los Angeles, California. "They'll be superfluous and they'll create great inequities and problems within the implementation, so I'm really concerned about the data for boosters in general."
One repeated sticking point for the CDC's panelists during deliberations on Thursday: the still-open question over whether boosting with mixed vaccines might be permitted -- since for those who received the Moderna or Johnson & Johnson COVID-19 vaccine, there is no third dose protection currently available.
The FDA's vaccine chief, Dr. Peter Marks, addressed the CDC's panelists ahead of Thursday's vote and acknowledged their frustrations.
"I think we understand at FDA the relative urgency here of trying to have a solution for anyone who has been vaccinated with any of the authorized or approved vaccines," Marks said. "Unfortunately, we're not in a place right now which I can give you an exact timeline, but I can tell you that we will proceed with all due urgency to try to get there as rapidly as possible."
ABC News' Eric M. Strauss contributed to this report.



