1st COVID-19 at home self-test authorized by FDA as cases surge in US
Google reported searches for the word "testing" hit an all-time high.
As the number of coronavirus cases across the U.S. continues to rise just days before Thanksgiving, Google is reporting the word "testing" has hit an all-time high on their search bar.
While many have turned to clinics to get tested for the virus, at home testing kits for COVID-19 are becoming more widely available too, and experts say they can be game changers, as they make it easier for people to find out if they're contagious.
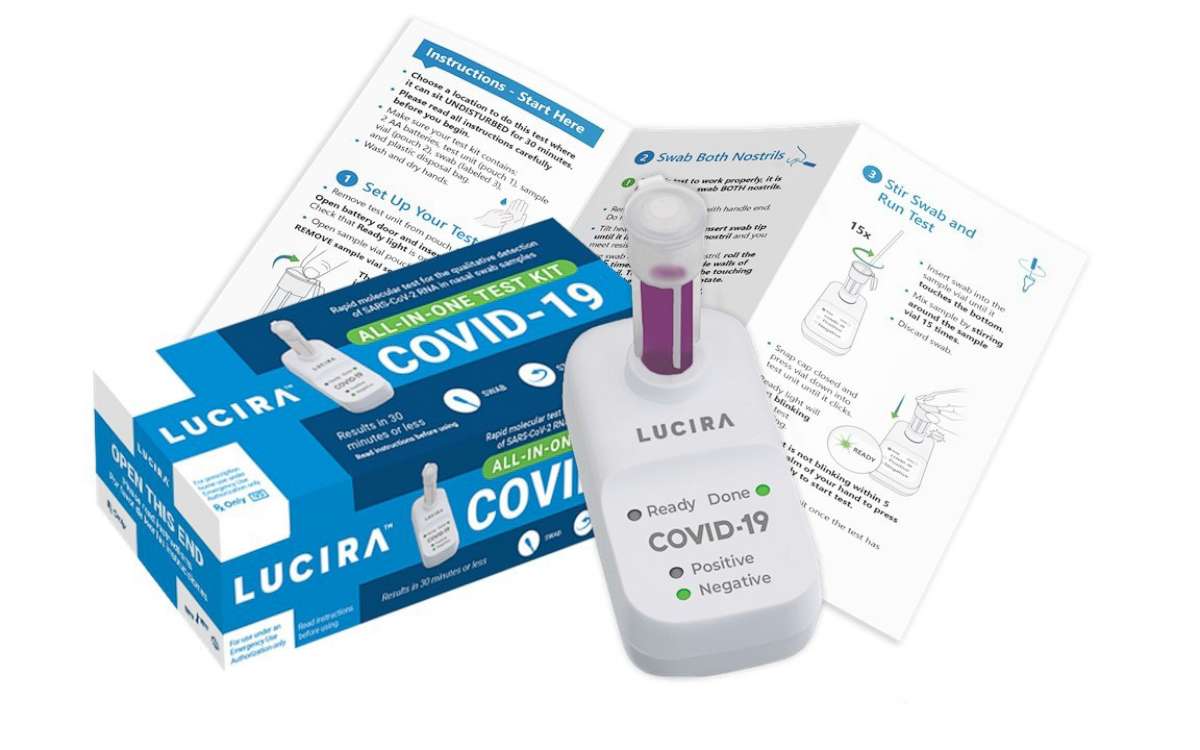
But on Tuesday, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the Lucira COVID-19 All-In-One Test Kit, the first COVID-19 diagnostic test for self-testing, which provides rapid results at home.
“While COVID-19 diagnostic tests have been authorized for at-home collection, this is the first that can be fully self-administered and provide results at home,” said FDA Commissioner Stephen M. Hahn, M.D., in a statement. “This new testing option is an important diagnostic advancement to address the pandemic and reduce the public burden of disease transmission.”

The Lucira COVID-19 All-In-One Test Kit is a self-collected nasal swab sample for individuals 14 and older, according to the FDA. In 30 minutes or less, the results can be read directly from the test unit’s light-up display that shows whether a person is positive or negative for SARS-CoV-2. The test can also be administered by health care providers.
The latest testing development is being described as a “significant step toward FDA’s nationwide response to COVID-19,” and a way for people to efficiently track and monitor results.
“More Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them,” said Jeff Shuren, M.D., J.D., the director of the FDA’s Center for Devices and Radiological Health, in the FDA news release about the test kit.
In addition to Lucira’s All-In-One test kit, several companies are also providing at-home tests, which allow people to take multiple tests a week as needed from the comfort of their homes. The down side is: Getting tested at home will cost you.
Costco, which is the latest to come out with at-home testing kits, is charging members $130 for a saliva test from Azova Health. Costco said the kit can only be purchased online and is available to store members in most states.
Despite the steep price of the testing kit, testing experts said PCR-based tests like Costco's are the most accurate kind available, as they have a reported accuracy of 95% and higher. All users have to do is spit into a vial and mail it in. Once the lab gets your saliva sample, it takes 12-72 hours to get your results, according to Costco and Azova Health.
Grocery chain Albertson's -- with stores like Safeway, Vons and Acme -- is also offering at home saliva-based tests from Phosphorus diagnostics. People can purchase a test for $139, and like Costco, does not require a customer to display symptoms or have known exposure to the virus to purchase.
"People who have that money to afford it, they will absolutely test before they see their older relative," Dr. Monica Gandhi, professor at the University of California, San Francisco School of Medicine, told "Good Morning America." "They will test before they get on a plane."
Beyond saliva tests, other companies like Walmart, which is working with Quest Labs, are offering at home nasal swab tests similar to what you'll find in your doctor's office and are delivering them to customers by drone.
In addition, Everlywell is offering at-home lower nasal swab kits for $109 and "LetsGetChecked" tells ABC News that their at-home lower nasal swab test kits are available for $119 and have been used at sporting events and movie studios to increase safety.
Almost all the companies, like Costco, say results will take 12 to 72 hours to turn around from the time the lab gets your kit, with many giving you overnight mailing labels so you can get your kits to the lab as quickly as possible.
People who use at-home testing for COVID-19 should still follow the recommended guidelines to prevent the spread of COVID-19, including social distancing and the wearing of face masks, health experts advise.
This report was featured in the Thursday, Nov. 19, 2020, episode of “Start Here,” ABC News’ daily news podcast.
"Start Here" offers a straightforward look at the day's top stories in 20 minutes. Listen for free every weekday on Apple Podcasts, Google Podcasts, Spotify, the ABC News app or wherever you get your podcasts.



