Trump, Fauci tout 'good news' from remdesivir drug trial in treating COVID-19
"What it has proven is that a drug can block this virus," Anthony Fauci said.
Infectious disease expert Dr. Anthony Fauci on Wednesday touted the results of trial examining an experimental drug treatment for the novel coronavirus, calling it "good news" as he spoke in the Oval Office alongside President Donald Trump.
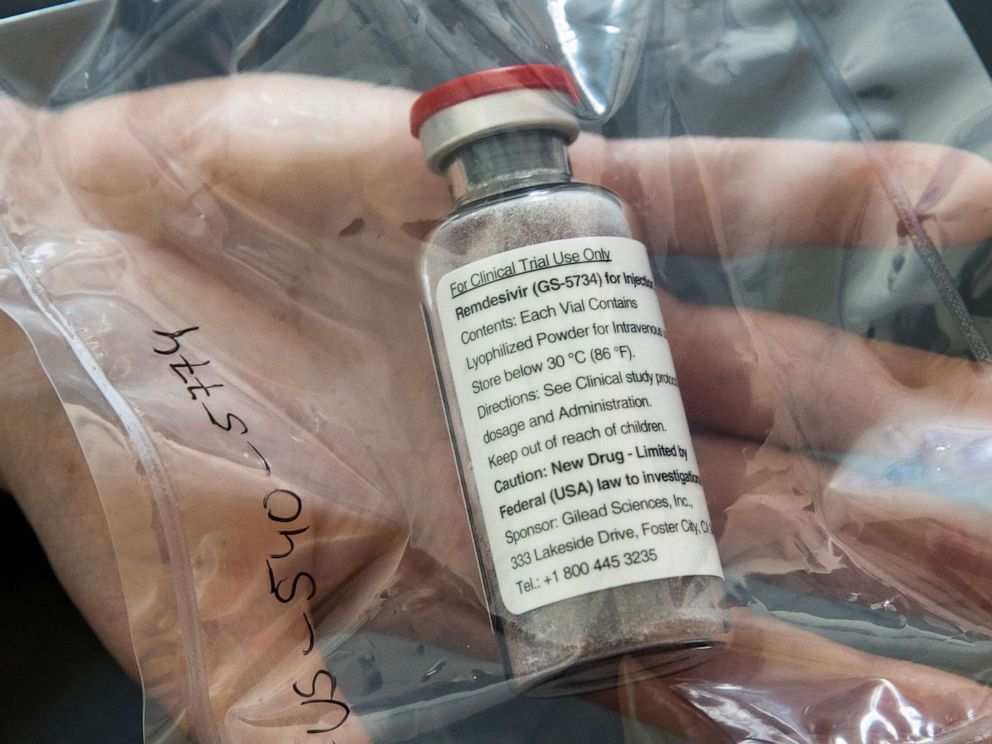
A randomized, international trial of the drug remdesivir had resulted in "quite good news," shortening the period patients experienced symptoms and potentially slightly reducing the mortality rate, according to Fauci, a member of the White House's coronavirus task force and the director of the National Institute of Allergy and Infectious Diseases, which sponsored the trial.
"What it has proven is that a drug can block this virus," Fauci said, calling the development "very optimistic."
Tune into ABC at 1 p.m. ET and ABC News Live at 4 p.m. ET every weekday for special coverage of the novel coronavirus with the full ABC News team, including the latest news, context and analysis.
The trial had 1,063 patients spread across 22 countries, including the U.S., and the first participant was an American who had been quarantined on the Diamond Princess, a cruise ship wracked by the virus that was docked in Japan earlier this year, according to the NIAID.
It had not yet been peer-reviewed but was being submitted to a journal for review, Fauci said as he previewed the results. Experts interviewed by ABC News urged caution until the full data was released.
Fauci said the data so far showed the drug, made by the biotech company Gilead Sciences, had "a clear-cut, significant positive effect in diminishing the time to recovery."
For those who took the drug, Fauci said, it took less time to recover, averaging 11 days compared to 15 days for those in a control group who received a placebo.
Fauci said the data represented "a very important proof of concept" -- showing that a drug could, in fact, "block" COVID-19.
He also said the mortality rate trended lower for those who took the drug -- 8% compared to 11% for those who did not -- although he noted that trend was not yet statistically significant, and the results will undergo further analysis.
Announcing the results was accelerated because of the ethical obligation to let patients in trial control groups know about this drug "so that they can have access," Fauci said.
Moving forward, he said, "this will be the standard of care."
The president, who in the past repeatedly encouraged COVID-19 patients to seek out a different, anti-malarial drug despite no strong evidence it helped, said that the results of the remdesivir trial were "good news." Trump has praised remdesivir in the past, too.
"It's a beginning, it means you build on it," Trump said Wednesday. "I love that as a building block -- you know, just as a building block, I love that. But certainly it's a positive, it's a very positive event from that standpoint."

Remdesivir, which is delivered through an intravenous infusion, was initially developed by Gilead to treat Ebola. Although initially promising, it didn’t prove as effective as other Ebola treatments, so research was halted.
However, laboratory studies found remdesivir might work against SARS, a close cousin to the virus that has caused the current COVID-19 pandemic. Because of its promise, governments around the world acted quickly to set up formal studies to answer the question: Does remdesivir help patients with COVID-19 get better faster?
Experts said today's results were hopeful but that more study was needed.
“The news about remdesivir might get us jumping for joy today but we need to see the data and continue to study this for a definitive answer," Jay Bhatt, an ABC News contributor who was until recently the chief medical officer of the American Hospital Association, noting the "rigor" of the study did give him "hope."
William Schaffner, a professor of medicine at Vanderbilt University and expert on infectious diseases, told ABC News the results were "very encouraging."
"These are the first data from a controlled trial," he said. "Even though the trial had to be truncated, this data would indicate we have a drug that can benefit patients. It’s not a miracle drug, but seems to reduce hospitalization duration and death rates. These are very, very important.”
Sony Salzman, Angela Baldwin and Dr. Chloë E. Nunneley contributed reporting.
What to know about coronavirus:
- How it started and how to protect yourself: coronavirus explained
- What to do if you have symptoms: coronavirus symptoms
- Tracking the spread in the US and Worldwide: coronavirus map
This report was featured in the Thursday, April 30, 2020, episode of “Start Here,” ABC News’ daily news podcast.
"Start Here" offers a straightforward look at the day's top stories in 20 minutes. Listen for free every weekday on Apple Podcasts, Google Podcasts, Spotify, the ABC News app or wherever you get your podcasts.




