AstraZeneca vaccine may slow virus transmission
For now, the vaccine is not available in the United States.
The AstraZeneca vaccine likely slows the transmission of the virus in addition to protecting against severe COVID-19 illness and death, according to a new study.
To come to that conclusion, University of Oxford researchers swabbed study participants in the U.K. every week and found a 67% reduction in positive swabs among participants who had been vaccinated.
"We haven’t specifically measured transmission because that’s a different type of study," Andrew Pollard, head of the Oxford Vaccine Group said during a Wednesday press briefing. "What we’ve got is a study which tells the number of people who are no longer infected and if you’re not infected there’s an assumption that you can’t then transmit the virus."
While the preliminary research was published in The Lancet Tuesday, the study itself has not yet been peer reviewed by external experts.
"The vaccine reduced by two-thirds the likelihood that people would carry, and hence be able to spread the virus," said Dr. William Schaffner, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center. "That’s a very exciting piece of information," he added.
"It’s the first solid information that a vaccine not only protects the individual, but reduces the spread of the virus from person to person," Schaffner said, which is key for eventually reaching herd immunity, where enough people have immunity that it affords protection to the rest of the population.
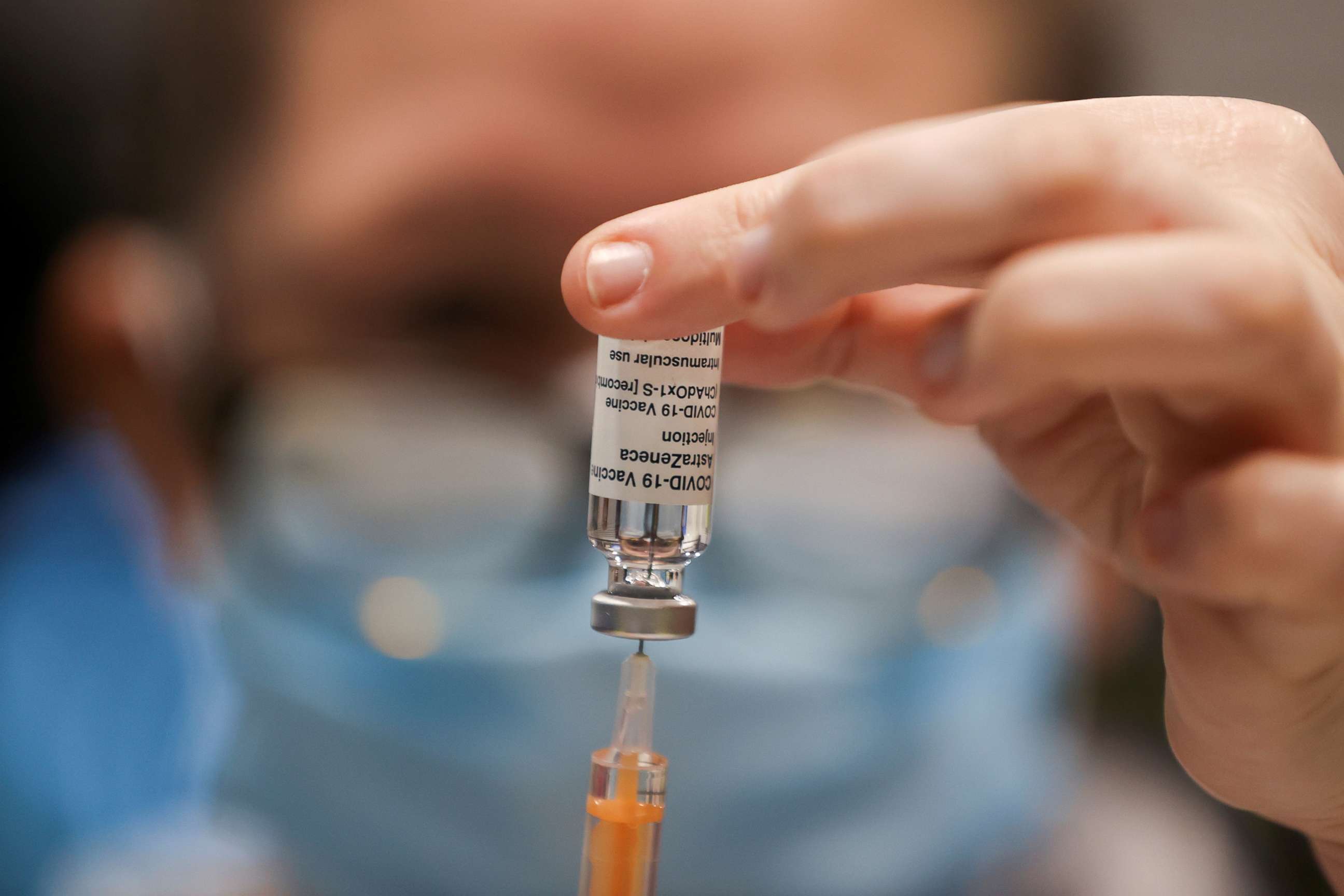
Although the new results are promising, the United States has opted not to move forward with the AstraZeneca vaccine until it has more data on it. The Food and Drug Administration has said that it is waiting to see results from a 30,000-person clinical trial that's ongoing in the U.S. before it moves forward with considering an emergency use authorization like it did for the Pfizer and Moderna vaccines. Data should be available by the end of the month.
The U.S. invested $1.5 billion to secure 300 million doses of the AstraZeneca vaccine last spring, assuming the vaccine is eventually authorized.
European countries have been similarly cautious about AstraZeneca's data, which has been criticized for lacking detail and transparency. On Wednesday, Switzerland declined to authorize the AstraZeneca vaccine, calling the data that's been submitted and analyzed so far "not yet sufficient."
"To obtain more information about safety, efficacy and quality, additional data from new studies are needed," the country's drug regulator, Swiss Agency for Therapeutic Products, said in a statement.
"Once those data are in we’ll have to look at them very, very carefully," Schaffner said. For now, he hopes the AstraZeneca news is a preview of what's to come for vaccines like Pfizer and Moderna. "We think it bodes well for the other vaccines, but we can’t say for sure," he added.
ABC News' Brian Hartman and Eric Strauss, Zoe Magee and Morgan Winsor contributed to this report.
What to know about the coronavirus:
- How it started and how to protect yourself: Coronavirus explained
- What to do if you have symptoms: Coronavirus symptoms
- Tracking the spread in the U.S. and worldwide: Coronavirus map
Tune into ABC at 1 p.m. ET and ABC News Live at 4 p.m. ET every weekday for special coverage of the novel coronavirus with the full ABC News team, including the latest news, context and analysis.