Senators, activists urge FDA to revise blood donation policy for gay, bisexual men amid coronavirus pandemic
Activists say lifting the policies could save more than one million lives.
Democratic senators and gay rights advocates are calling on the federal government to loosen restrictions on blood donations from gay and bisexual men, citing the recent blood shortages caused by the novel coronavirus pandemic as a catalyst for change.
The Food and Drug Administration's current recommendations restrict men who have sex with men, commonly referred to as MSM, from donating blood within 12 months of their last sexual encounter. The policy harkens back to the HIV/AIDS crisis of the 1980s, which disproportionately impacted MSM.
While this policy is a more relaxed version of the previous outright ban on all MSM donations, many are hoping blood screenings and advanced technology could prompt the agency to rethink its regulations.
In a letter to FDA Commissioner Stephen Hahn, 17 Democratic senators urged the agency to update its 12-month deferral policy, calling it "stigmatizing" and "discriminatory."
"As such, it is imperative that we move away from discriminatory donor deferral policies that prohibit many healthy individuals from contributing much-needed blood and blood products," the letter reads.
Instead, they're asking the FDA to base blood donation eligibility on individualized situations rather than "inaccurate stereotypes."
"We are steadfastly committed to ending this policy and encourage the FDA to shift to scientific practices that secure our nation's blood supply based on individual risk rather than the perpetuation of inaccurate stereotypes," the senators wrote.
Tune into ABC at 1 p.m. ET and ABC News Live at 4 p.m. ET every weekday for special coverage of the novel coronavirus with the full ABC News team, including the latest news, context and analysis.
Dr. Jeff Kirchner, the chief medical officer at the American Academy of HIV Medicine and a practicing HIV specialist, explained that after adhering to antiviral therapy for a number of months, HIV patients who have undetectable loads of the virus in their blood are no longer contagious. The National Institute of Health calls this concept "undetectable equals untransmittable."
"We know that patients who are on therapy and have undetectable viral loads can't transmit their virus, and whether it would be through a blood transfusion, a needle stick, a sexual partner," Kirchner told ABC News.
But even so, Kirchner said that all blood, regardless of the donor, is screened for a list of diseases -- including HIV-1 and HIV-2.
"Everybody's blood is screened for these transmissible infectious diseases, regardless of who the donor is: a gay woman, a straight woman, a gay man, a bisexual man," Kirchner said. "All the donated blood is appropriately screened for contagious infections."
For those reasons, Kirchner feels that the benefits of MSM transfusions outweigh the risks.
"I'm all about the science and following the science, but I think in this case again the science is strong regarding the transmissibility of it," Kirchner said. "I think that I would have to say that this is truly a situation where the benefits greatly outweigh the risk."
That risk, however, is the period of time between when a person may have been exposed to HIV, and when a test can determine if an individual has the virus. This "window period," according to the Centers for Disease Control and Prevention, can range anywhere from 10 days after exposure to up to three months after.
"So that window period theoretically could be missed if somebody, again, had sex with somebody, they got infected, and then went out and donated blood two days later. Their blood would not show any evidence of HIV, whether it was through specific viral load testing, or certainly not antibody," Kirchner said.
However, according to Kirchner, it's a "small window where they could possibly slip through."
GLAAD, an LGBTQ advocacy group, is also pushing the FDA to change its policy. The organization started a petition, "lift the ban on gay, bisexual, and MSM from donating blood," that now has over 15,000 signatures, some from celebrities like singer Sam Smith. They are also calling the FDA's policy "discriminatory" and "antiquated."
"This is outdated and it stigmatizes gay and bisexual men," GLAAD President and CEO Sarah Kate Ellis told ABC News' Lindsey Davis.
She continued, "And I think that what this does is just reinforce the notion that there's something wrong with being gay or being bisexual, and that is not a good thing, especially when medical medicine has far surpassed that notion."
Ellis explained that according to a 2014 study by the Williams Institute, if the MSM ban were lifted, more than 600,000 additional pints of blood would be donated, which could help save the lives of more than a million individuals.
The Human Rights Campaign, the largest LGBTQ civil rights organization in the U.S., is also calling on the FDA to change its policy for MSM blood donations. In a letter sent Thursday to Commissioner Hahn, the group pointed out testing technologies that reduce the window period for diseases -- including HIV -- and like the senators, called for donation eligibility to be determined on an individualized basis rather than excluding an entire group.
"It is critical that deferral be based on information that is within the personal knowledge and control of the prospective donor -- and not on the sexual orientation or gender identity of the donor, the sexual orientation, gender identity, or activities of one’s sexual partners, or on perceived monogamy,” Alphonso B. David, President of the organization, wrote in the letter.
He continued, "A policy focused on the prospective donor’s activity, rather than identity, will not only be safer but rational."
The senators, GLAAD and the Human Rights Campaign all cited the COVID-19 outbreak and recent blood shortages as reasons to call for action now. On March 17, the American Red Cross announced a "severe blood shortage," explaining that social distancing guidelines and coronavirus fears led many blood drives to be canceled, resulting in fewer blood donations.
"In light of this shortage, we urge you to swiftly update blood donor deferral policies in favor of ones that are grounded in science, are based on individual risk factors, do not unfairly single out one group of individuals, and allow all healthy Americans to donate," the senators wrote.
While the Red Cross has since said that the blood supply has been replenished from "concerning low levels," there are concerns about the future.
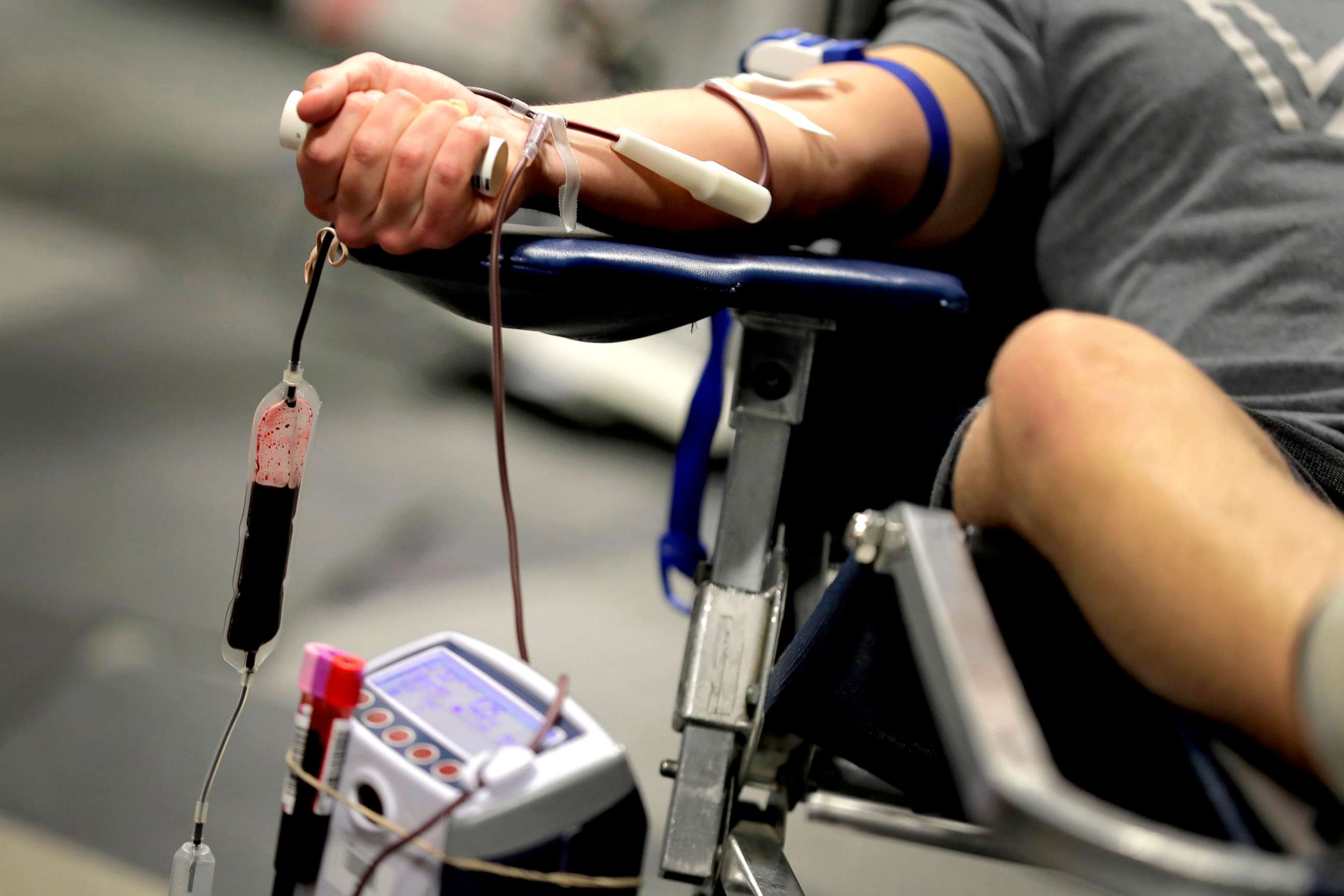
"And right now, as I said, things are stable. But we don't know what next week is going to bring," Kristal Baker, the manager for transfusion safety, quality surveillance and analysis for the Piedmont HealthCare System in Atlanta told ABC News.
The American Red Cross, America's Blood Centers and the American Association of Blood Banks also support an update to the current policy. The Red Cross is specifically advocating for the deferral time to be reduced from 12 months to three months.
When asked for comment, an FDA spokesperson cited the recent decline in blood and plasma donations then wrote, "At this time, FDA's recommendations regarding blood donor deferral for men who have sex with men have not changed, but we are actively considering the situation as the outbreak progresses."
At the FDA's Blood Products Advisory Committee meeting on March 21, 2019, an FDA official said the "FDA is committed to ongoing evaluation of the MSM 12-month deferral policy and potentially advancing the policy based on the available scientific evidence."
What to know about coronavirus:
- How it started and how to protect yourself: coronavirus explained
- What to do if you have symptoms: coronavirus symptoms
- Tracking the spread in the US and Worldwide: coronavirus map




