Second Birth Control Pill Recall in Month
Glenmark Generics recalled seven lots of generic birth control pills because of a packaging error. (FDA)
Less than one month after Pfizer recalled nearly 1 million packages of faulty birth control pills, Glenmark Generics has recalled its version of the oral contraceptives because of a packaging error that landed the pills in the wrong order.
The India-based drug company has warned that seven lots of generic norgestimate and ethinyl estradiol tablets distributed to U.S. pharmacies between Sept. 21 and Dec. 30, 2011, "could leave women without adequate contraception, and at risk for unintended pregnancy."
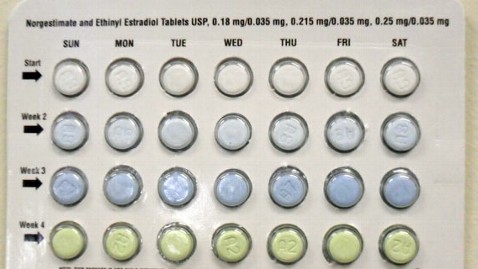
"Select blisters were rotated 180 degrees within the card, reversing the weekly tablet orientation and making the lot number and expiry date visible only on the outer pouch," the company said in a statement released Friday, explaining the packaging error.
The correct packaging aligns 28 tablets in four rows, with the white tablets containing norgestimate and ethinyl estradiol in the top row and light green placebo tablets in the bottom row.
The affected lot numbers are 04110101, 04110106, 04110107, 04110114, 04110124, 04110129 and 04110134.
"Patients who have the affected product should notify their physician and return the product to the pharmacy" and "begin using a nonhormonal form of contraception immediately," the company said.