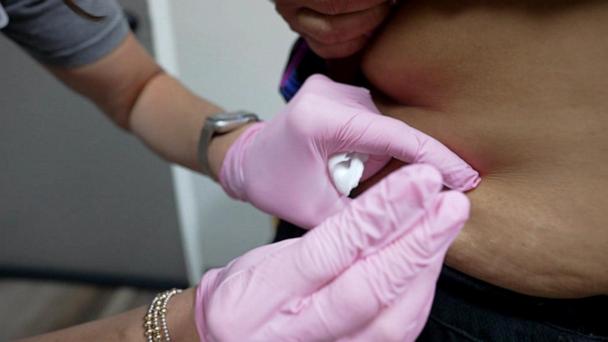
FDA expected to authorize Moderna COVID-19 vaccine after panel recommendation
The Moderna vaccine would be the second to receive emergency use authorization in the U.S.
December 18, 2020
Additional Live Streams
Additional Live Streams
 Live
LiveABC News Live
 Live
LivePres. Trump hosts Black History Month event at the White House
 Live
LiveSenate holds confirmation vote for FBI director nominee Kash Patel
 Live
LiveMount Kilauea erupts in Hawaii
 Live
LiveHospital in Rome where Pope Francis is hospitalized for bronchitis
 Live
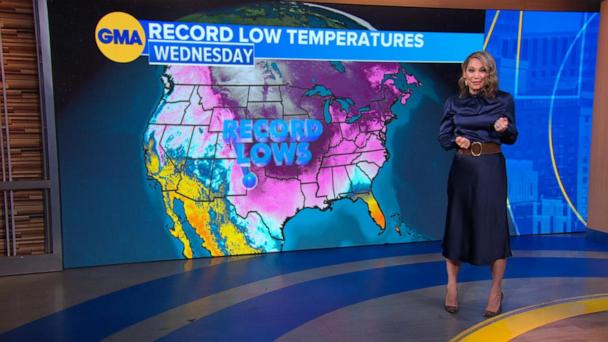
LiveWarmer weather moves into the Heartland this weekend; temperatures rise in Northeast next week
 Live
LiveDow Jones Industrial Average
Top Stories
Top Stories
A look inside the presidential descendants club
2 hours agoEnvironment can have greater impact on health than genes, study shows
2 hours agoMitch McConnell announces he will not seek reelection next year
3 hours agoDangerous winter storm bringing heavy snow and brutal cold
Feb 20, 2025Millions hit by brutal cold as winter weather sweeps country
Feb 20, 2025Trump attacks Zelenskyy amid US talks with Russia
Feb 20, 2025Russia launches 'massive' attack into Ukraine amid Trump-Zelenskyy dispute
Feb 20, 2025Joint Chiefs Chairman Brown on list to possibly be removed from post by Hegseth
3 hours agoBodies of 4 hostages, believed to include Bibas family, released by Hamas
Feb 20, 2025Delta offers $30K to passengers on jet that flipped on Toronto runway
Feb 20, 2025IRS and Defense Department face layoffs and budget cuts
Feb 20, 2025Hochul ‘in fight mode’ over Trump’s move to stop NYC congestion pricing
Feb 20, 2025Judge denies request to exclude evidence at Idaho murder trial
Feb 20, 2025Education department gives schools 2 weeks to ban DEI initiatives
Feb 20, 2025US allies and Ukraine react to Trump’s shift towards Russia
Feb 20, 20252 girls arrested for planning 'mass casualty attack'
Feb 20, 2025Grand jury says police department should be 'abolished' as 5 officers charged
Feb 20, 2025Justin Baldoni fires back after Blake Lively files amended complaint
Feb 20, 2025WWII veteran celebrates 100th birthday
Feb 20, 2025Apple unveils iPhone 16e
Feb 20, 202510 days away from Oscars, hosted by Conan O'Brien
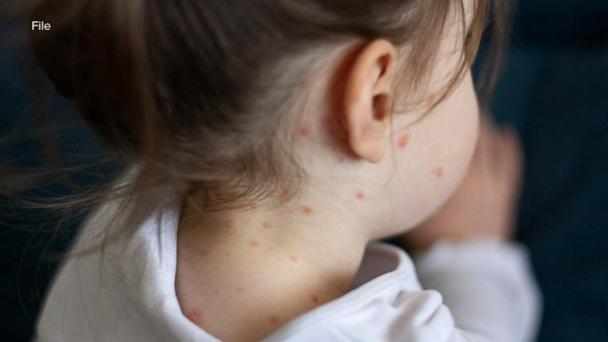
Feb 20, 2025Texas' measles outbreak spreads to New Mexico
Feb 20, 2025Father saves son after suffering cardiac arrest — here’s what you can learn from it
Feb 20, 2025Researchers working to explain why more young people are getting cancer
Feb 20, 2025Emma Radacanu cries on tennis court during security scare
Feb 20, 2025Could a personalized vaccine help fight pancreatic cancer?
Feb 20, 2025Celebrating 12 years since Robin Roberts' 'GMA' return
Feb 20, 2025Reeve Hockey Classic celebrates para-athletes
Feb 20, 2025Mother charged for allegedly abandoning children 'in deplorable conditions' for years
Feb 20, 2025'I was dead for 90 seconds’: Man saved after heart stops at basketball game
Feb 20, 2025
ABC News Live Presents
ABC News Live Presents
America’s Care Crisis
Feb 14, 2025Immigration Crackdown
Feb 13, 2025Shot in the Dark: Weight Loss Injection Wars
Nov 26, 2024October 7th: The Race to Survive
Oct 08, 2024January 6th: The Fight to Rewrite History
Oct 04, 2024The President and First Lady of Ukraine | Robin Roberts Reporting
Sep 26, 2024Maui Strong 808: Rising from the Ashes
Aug 09, 202410 Million Names
Jun 20, 2024Generation Swipe
May 24, 2024Amplified: Asian American Native Hawaiian Pacific Islander Voices
May 17, 2024The Power of Us: People, Climate and Our Future
Apr 26, 2024Trashed: The Secret Life of Plastic Exports
Apr 24, 2024Toll of War: The José Andrés Interview | Martha Raddatz Reports
Apr 09, 2024Fertility in America | Rebecca Jarvis Reporting
Mar 29, 2024One-on-One: A Conversation with Robin Roberts and Caitlin Clark
Mar 16, 2024After the Fall: A Conversation with Robin Roberts and Jenifer Lewis
Mar 13, 2024Prince Harry's Mission: Life, Family and Invictus Games
Feb 24, 2024Tackling Mental Health | Michael Strahan Reports
Feb 17, 2024Severed: Diabetes Denial and Mistrust
Feb 16, 2024Exodus: Global Migration
Jan 27, 2024Battle Cry: Fighting Assault in the Military
Dec 08, 2023Fallout: Two Nations Under Uranium
Nov 30, 2023The American Classroom
Nov 18, 2023Disaster Uninsured
Nov 17, 2023Hispanic Heritage Month: Entre Nos - 1st Gen
Sep 29, 2023Hispanic Heritage Month: Entre Nos – 2nd Gen
Oct 10, 2023Hip-hop at 50: The architect, the First Amendment and the fashion explosion
Aug 24, 2023Elliot Page: In His Own Words
Jun 20, 2023Culture Conversations - CC: AANHPI Heritage Month
May 25, 2023Trashed: The Secret Life of Plastic Recycling
May 24, 2023
ABC News Specials on
Impact X Nightline: On the Brink
Dec 14, 2023Impact X Nightline: Unboxing Shein
Nov 27, 2023The Lady Bird Diaries
Nov 27, 2023Impact X Nightline: It's Britney
Nov 27, 2023Impact X Nightline: Natalee Holloway -- A Killer Confesses
Nov 27, 2023Impact X Nightline: Who Shot Tupac?
Nov 27, 2023Wild Crime
Oct 26, 2022Impact x Nightline
Oct 28, 2022Power Trip: Those Who Seek Power and Those Who Chase Them
Sep 27, 2022The Murders Before the Marathon
Sep 01, 2022The Ivana Trump Story: The First Wife
Jul 25, 2022Aftershock
Jul 18, 2022Mormon No More
Jun 22, 2022Leave No Trace: A Hidden History of the Boy Scouts
Jun 15, 2022Keeper of the Ashes: The Oklahoma Girl Scout Murders
May 20, 2022The Orphans of COVID: America's Hidden Toll
May 13, 2022Superstar: Patrick Swayze
Apr 14, 2022The Kardashians -- An ABC News Special
Apr 05, 202224 Months That Changed the World
Mar 30, 2022Have You Seen This Man?
Mar 22, 2022