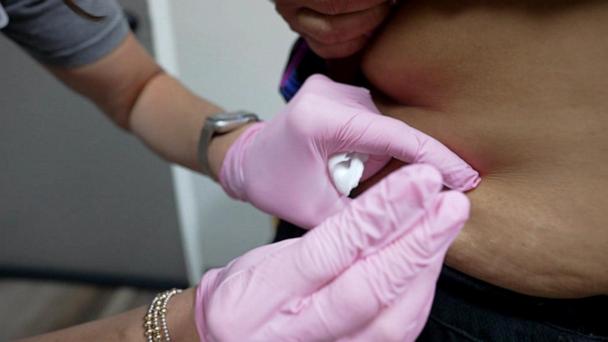
Doctor receives new FDA-authorized coronavirus treatment
David Schulz, a doctor who treats COVID-19 patients, received Eli Lilly’s new FDA-authorized antibody treatment, Bamlanivimad, while being treated in Indiana.
November 12, 2020
Additional Live Streams
Top Stories
Top Stories
3 years of brutal war in Ukraine
Feb 25, 2025What one Palestinian woman finds upon her return to northern Gaza
Feb 25, 2025Trump defends Musk's email ultimatum to federal workers
Feb 25, 2025Pope Francis has seen 'slight improvement', condition remains critical: Vatican
Feb 25, 2025Divisions remain on how to end Russia-Ukraine war on its 3rd anniversary
Feb 25, 2025New coronavirus found in bats, but not currently 'concern to public health': CDC
Feb 25, 2025Suspect in deadly Pennsylvania hospital hostage standoff wanted revenge: Sources
Feb 25, 2025Egg prices soar across US as Dennys adds surcharge
Feb 25, 2025Cabin smoke on Delta plane forces passengers to evacuate
Feb 25, 2025Iconic singer Roberta Flack dies at the age of 88
Feb 25, 2025Security concerns over DOGE access to confidential data grow
Feb 24, 2025Diddy’s lawyers ask judge to suppress evidence
Feb 24, 2025Trump, Macron discuss Ukraine in Oval Office meeting
Feb 24, 2025Claims Ukraine started war are ‘complete and utter nonsense’: Boris Johnson
Feb 24, 2025What US troops in eastern Europe are learning from the war in Ukraine
Feb 24, 2025Ruby Franke's estranged husband warns about social media dangers
Feb 24, 2025Thieves fake medical emergency, steal puppies from pet store
Feb 24, 2025Recapping awards show weekend
Feb 24, 2025Things ER doctors wish you would avoid
Feb 24, 2025Hostage video showing sign of life is ‘red flag’ to save them: Family
Feb 24, 2025Virginia Beach police chief says 2 of his officers were 'executed'
Feb 24, 2025Sen. Richard Blumenthal talks federal firings
Feb 24, 2025Dan Bongino named as deputy FBI director
Feb 24, 2025Trump administration calls for ICE agents to locate migrant children: Source
Feb 24, 2025Supreme Court hears arguments in Texas death row case
Feb 24, 2025Germany to move toward 'independence' from US, new leader says
Feb 24, 2025Humanitarian challenges after phase 1 of ceasefire in Gaza
Feb 24, 2025Elon Musk delivers ultimatums to federal workers as deadline approaches
Feb 24, 2025Trump guts senior leadership at Pentagon
Feb 24, 2025American Airlines diverted to Rome after fake bomb threat
Feb 24, 2025
ABC News Live Presents
ABC News Live Presents
America’s Care Crisis
Feb 14, 2025Immigration Crackdown
Feb 13, 2025Shot in the Dark: Weight Loss Injection Wars
Nov 26, 2024October 7th: The Race to Survive
Oct 08, 2024January 6th: The Fight to Rewrite History
Oct 04, 2024The President and First Lady of Ukraine | Robin Roberts Reporting
Sep 26, 2024Maui Strong 808: Rising from the Ashes
Aug 09, 202410 Million Names
Jun 20, 2024Generation Swipe
May 24, 2024Amplified: Asian American Native Hawaiian Pacific Islander Voices
May 17, 2024The Power of Us: People, Climate and Our Future
Apr 26, 2024Trashed: The Secret Life of Plastic Exports
Apr 24, 2024Toll of War: The José Andrés Interview | Martha Raddatz Reports
Apr 09, 2024Fertility in America | Rebecca Jarvis Reporting
Mar 29, 2024One-on-One: A Conversation with Robin Roberts and Caitlin Clark
Mar 16, 2024After the Fall: A Conversation with Robin Roberts and Jenifer Lewis
Mar 13, 2024Prince Harry's Mission: Life, Family and Invictus Games
Feb 24, 2024Tackling Mental Health | Michael Strahan Reports
Feb 17, 2024Severed: Diabetes Denial and Mistrust
Feb 16, 2024Exodus: Global Migration
Jan 27, 2024Battle Cry: Fighting Assault in the Military
Dec 08, 2023Fallout: Two Nations Under Uranium
Nov 30, 2023The American Classroom
Nov 18, 2023Disaster Uninsured
Nov 17, 2023Hispanic Heritage Month: Entre Nos - 1st Gen
Sep 29, 2023Hispanic Heritage Month: Entre Nos – 2nd Gen
Oct 10, 2023Hip-hop at 50: The architect, the First Amendment and the fashion explosion
Aug 24, 2023Elliot Page: In His Own Words
Jun 20, 2023Culture Conversations - CC: AANHPI Heritage Month
May 25, 2023Trashed: The Secret Life of Plastic Recycling
May 24, 2023
ABC News Specials on

Impact X Nightline: On the Brink
Dec 14, 2023
Impact X Nightline: Unboxing Shein
Nov 27, 2023
The Lady Bird Diaries
Nov 27, 2023
Impact X Nightline: It's Britney
Nov 27, 2023
Impact X Nightline: Natalee Holloway -- A Killer Confesses
Nov 27, 2023
Impact X Nightline: Who Shot Tupac?
Nov 27, 2023
Wild Crime
Oct 26, 2022
Impact x Nightline
Oct 28, 2022
Power Trip: Those Who Seek Power and Those Who Chase Them
Sep 27, 2022
The Murders Before the Marathon
Sep 01, 2022
The Ivana Trump Story: The First Wife
Jul 25, 2022
Aftershock
Jul 18, 2022
Mormon No More
Jun 22, 2022
Leave No Trace: A Hidden History of the Boy Scouts
Jun 15, 2022
Keeper of the Ashes: The Oklahoma Girl Scout Murders
May 20, 2022
The Orphans of COVID: America's Hidden Toll
May 13, 2022
Superstar: Patrick Swayze
Apr 14, 2022
The Kardashians -- An ABC News Special
Apr 05, 2022
24 Months That Changed the World
Mar 30, 2022
Have You Seen This Man?
Mar 22, 2022