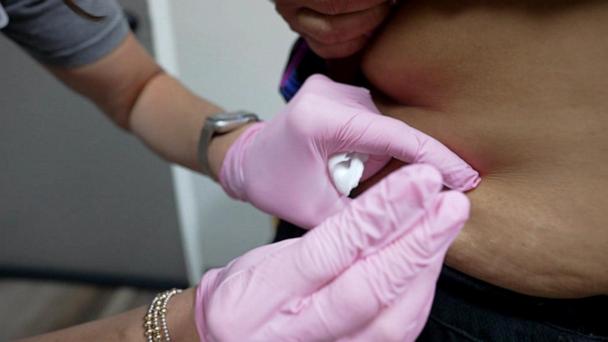
FDA authorizes emergency convalescent plasma use for COVID-19 patients
"Scientists have concluded that COVID-19 convalescent plasma is safe and shows promising efficacy," the FDA commissioner, Dr. Stephen Hahn, said during a White House briefing.
August 23, 2020
Additional Live Streams
Additional Live Streams
Top Stories
Top Stories
Severe weather wreaks havoc from the Gulf Coast to New England
3 hours agoRubio meets with Israeli Prime Minister Benjamin Netanyahu
3 hours agoJudges fired amid immigration crackdowns
3 hours agoMusk starts mass government layoffs, targets roughly 200,000 employees
Feb 16, 2025Auburn outfielder honors late mother after hitting home run
3 hours agoWhat to know about the bird flu outbreak and its risk to your health and wallet
Feb 16, 2025Luigi Mangione releases statement from prison as he faces court this week
Feb 16, 2025How deep are the government cuts likely to go?
Feb 16, 2025Mayor Adams’ cooperation with Trump administration is 'very disturbing': Jeffries
Feb 16, 2025Are the BAFTAS the true predictor of Oscar things to come?
Feb 16, 2025Revving up for Daytona 500
Feb 16, 2025Marco Rubio meets with Benjamin Netanyahu
Feb 16, 2025Drivers flee after deadly pileup on major highway
Feb 16, 2025Jane Doe drops lawsuit against Jay-Z, Diddy
Feb 16, 2025Pope spends 2nd night in hospital for bronchitis treatment
Feb 16, 2025American hostage reunites with family after release from Hamas
Feb 16, 2025Measles rapidly spreading through rural Texas
Feb 16, 2025DOJ files motion to dismiss charges against NYC mayor
Feb 15, 2025Thousands of federal workers fired in latest layoffs
Feb 15, 2025Brother of Israeli hostage on recent hostage release
Feb 15, 2025How dismantling the Department of Education could impact millions of students
Feb 15, 2025Egg prices hit record high
Feb 16, 2025US officials to start Russia-Ukraine negotiations
Feb 16, 2025Fear continues to ripple through immigrant communities
Feb 15, 2025Hamas releases 3 more hostages, including American Sagui Dekel Chen
Feb 15, 2025Special Report: Hamas escorts 3 hostages onto stage prior to release
Feb 15, 2025Pope Francis in the hospital
Feb 15, 2025A look at Trump's efforts to slim down the government and foreign policy
Feb 15, 2025New round of mass layoffs underway for federal workers
Feb 15, 2025Black box reveals new details in deadly DC crash
Feb 15, 2025
ABC News Live Presents
ABC News Live Presents
America’s Care Crisis
Feb 14, 2025Immigration Crackdown
Feb 13, 2025Shot in the Dark: Weight Loss Injection Wars
Nov 26, 2024October 7th: The Race to Survive
Oct 08, 2024January 6th: The Fight to Rewrite History
Oct 04, 2024The President and First Lady of Ukraine | Robin Roberts Reporting
Sep 26, 2024Maui Strong 808: Rising from the Ashes
Aug 09, 202410 Million Names
Jun 20, 2024Generation Swipe
May 24, 2024Amplified: Asian American Native Hawaiian Pacific Islander Voices
May 17, 2024The Power of Us: People, Climate and Our Future
Apr 26, 2024Trashed: The Secret Life of Plastic Exports
Apr 24, 2024Toll of War: The José Andrés Interview | Martha Raddatz Reports
Apr 09, 2024Fertility in America | Rebecca Jarvis Reporting
Mar 29, 2024One-on-One: A Conversation with Robin Roberts and Caitlin Clark
Mar 16, 2024After the Fall: A Conversation with Robin Roberts and Jenifer Lewis
Mar 13, 2024Prince Harry's Mission: Life, Family and Invictus Games
Feb 24, 2024Tackling Mental Health | Michael Strahan Reports
Feb 17, 2024Severed: Diabetes Denial and Mistrust
Feb 16, 2024Exodus: Global Migration
Jan 27, 2024Battle Cry: Fighting Assault in the Military
Dec 08, 2023Fallout: Two Nations Under Uranium
Nov 30, 2023The American Classroom
Nov 18, 2023Disaster Uninsured
Nov 17, 2023Hispanic Heritage Month: Entre Nos - 1st Gen
Sep 29, 2023Hispanic Heritage Month: Entre Nos – 2nd Gen
Oct 10, 2023Hip-hop at 50: The architect, the First Amendment and the fashion explosion
Aug 24, 2023Elliot Page: In His Own Words
Jun 20, 2023Culture Conversations - CC: AANHPI Heritage Month
May 25, 2023Trashed: The Secret Life of Plastic Recycling
May 24, 2023
ABC News Specials on

Impact X Nightline: On the Brink
Dec 14, 2023
Impact X Nightline: Unboxing Shein
Nov 27, 2023
The Lady Bird Diaries
Nov 27, 2023
Impact X Nightline: It's Britney
Nov 27, 2023
Impact X Nightline: Natalee Holloway -- A Killer Confesses
Nov 27, 2023
Impact X Nightline: Who Shot Tupac?
Nov 27, 2023
Wild Crime
Oct 26, 2022
Impact x Nightline
Oct 28, 2022
Power Trip: Those Who Seek Power and Those Who Chase Them
Sep 27, 2022
The Murders Before the Marathon
Sep 01, 2022
The Ivana Trump Story: The First Wife
Jul 25, 2022
Aftershock
Jul 18, 2022
Mormon No More
Jun 22, 2022
Leave No Trace: A Hidden History of the Boy Scouts
Jun 15, 2022
Keeper of the Ashes: The Oklahoma Girl Scout Murders
May 20, 2022
The Orphans of COVID: America's Hidden Toll
May 13, 2022
Superstar: Patrick Swayze
Apr 14, 2022
The Kardashians -- An ABC News Special
Apr 05, 2022
24 Months That Changed the World
Mar 30, 2022
Have You Seen This Man?
Mar 22, 2022